ID: PMRREP33045| 145 Pages | 6 May 2025 | Format: PDF, Excel, PPT* | Healthcare

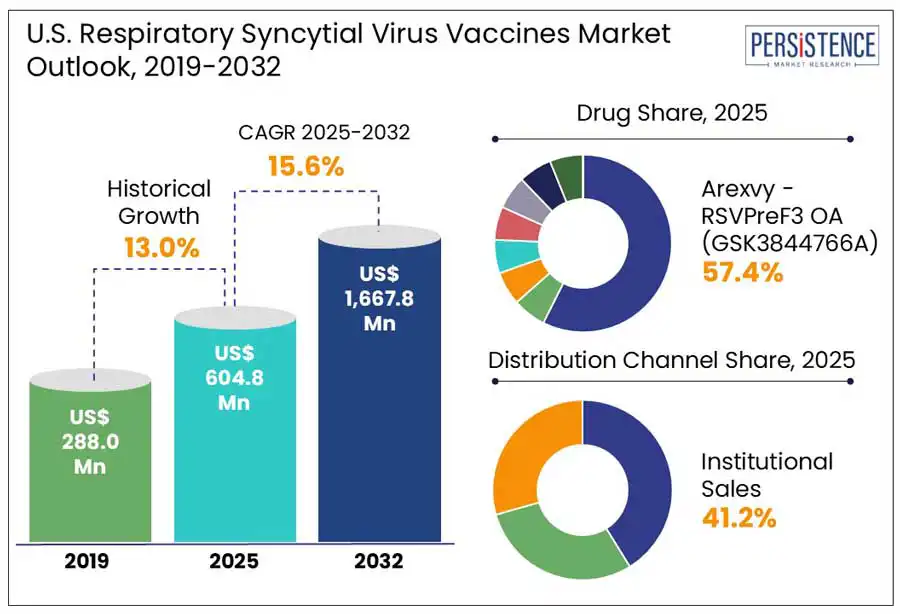
The U.S. respiratory syncytial virus market size is predicted to be valued at US$ 604.8 Mn in 2025. In-depth industry analysis reveals that respiratory syncytial virus vaccine sales in the U.S. are expected to surge at 15.6% CAGR and reach a market valuation of US$ 1,667.8 Mn by the end of 2032. U.S. sales of respiratory syncytial virus vaccines accounted for nearly 5% share of the US$ 9.0 Bn global respiratory virus vaccines market in 2024.
|
Market Attribute |
Key Insights |
|
U.S. Respiratory Syncytial Virus Vaccines Market Size (2025E) |
US$ 604.8 Mn |
|
Market Value Forecast (2032F) |
US$ 1,667.8 Mn |
|
Projected Growth (CAGR 2025 to 2032) |
15.6% |
|
Historical Market Growth (CAGR 2019 to 2024) |
13.0% |
Respiratory syncytial virus is a common respiratory virus that generally causes mild, cold-like symptoms. Demand for respiratory syncytial virus vaccines is expected to surge owing to rising disease burden of the causal pathogen, the respiratory syncytial virus. Infection by the respiratory syncytial virus may lead to severe respiratory diseases such as bronchiolitis and pneumonia, which can further progress into respiratory failure or death in rare cases. Respiratory Syncytial Virus (RSV) is also one of the major causes of hospitalizations and deaths in the elderly adult population in the U.S.
RSV vaccines for adults are gaining recognition for the prevention of pneumonia and exacerbations of chronic pulmonary or cardiac disease. Every year during fall, winter, and spring, RSV infections find prevalence within most regions of the U.S. This gives rise to the sales growth of RSV drugs to treat the large U.S. population from seasonal RSV.
High rates of mortality associated with RSV have given rise to a surge in the demand for respiratory syncytial virus prophylaxis, which is set to drive the growth of the U.S. respiratory syncytial virus vaccines market, owing to the rising prevalence of RSV-associated respiratory diseases. Furthermore, RSV is a major cause of morbidity and mortality in premature infants and infants with bronchopulmonary dysplasia. As per the recent study, hospitalization rates for RSV among children aged less than 1 year are around 16X greater than those for influenza. Incidence of RSV hospitalization is around 25.9/1000 infants at 1 month of age and 5.2/1000 among all infants in the age group younger than 2 years, in the U.S.
As such, the increasing rate of premature births is contributing to the rising demand for RSV prophylaxis, owing to RSV being more commonly found in infants as compared to adults. In March 2025, the World Health Organization (WHO) granted prequalification of the first maternal RSV vaccine, aimed at protecting infants from one of the leading causes of acute lower respiratory infections in children worldwide.
As per a detailed analysis of the U.S. respiratory syncytial virus vaccines market by Persistence Market Research, the industry stood at around US$ 533.1 Mn in 2024, mainly on the back of high sales of Palivizumab for serious lower respiratory tract infections caused by the respiratory syncytial virus. Palivizumab is a humanized monoclonal antibody that protects from infection caused by a respiratory syncytial virus.
Thus, demand for Palivizumab is expected to rise over the coming years owing to rising rate of respiratory tract infections in the U.S.
Collaborative efforts by key players aim toward launching an efficient and cost-effective molecule to prevent RSV disease. It is anticipated to positively affect the U.S. respiratory syncytial virus vaccines value during the forecast period.
The U.S. government has displayed a working collaboration with the industry to support its growth. The federal government of the United States has played a crucial role in the early development of a number of industries, not only through research and development activities, but also through provisions of monetary support for dormant businesses and government accession. This approach is set to provide lucrative growth opportunities for respiratory syncytial virus vaccine suppliers.
Additionally, government agencies are now included within the list of pre-clinical vaccine developers along with existing pharmaceutical players. Funding and support from the government is expected to create lucrative opportunities for market growth.
Furthermore, key manufacturers of respiratory syncytial virus vaccines are focused on reducing the cost of treatment for RSV-associated infections by investing in the development of novel mechanisms to enhance drug efficacy and reduce vaccine cost.
These rising trends in the application of novel mechanisms for the treatment of RSV-associated infections is expected to propel the sales growth of respiratory syncytial virus vaccines throughout the forecasted years.
In addition, the chances of contracting RSV-associated infections are high in immunocompromised hosts and can be cured by ribavirin treatment for respiratory syncytial virus.
A collaborative approach toward discovering therapeutics for RSV infection is a predominant trend experienced in the market space, which is set to enhance the sales of RSVC drugs and provide lucrative opportunities for market players.
The development of efficient, effective, as well as safe vaccines is a global health necessity, owing to the increasing disease burden of RSV. The growing number of RSV vaccine candidates in varied formats, such as subunit vaccines, live-attenuated vaccines, particle-based vaccines, and vector-based vaccines, is getting established and is currently present at different phases, many of them being in the clinical stage of development.
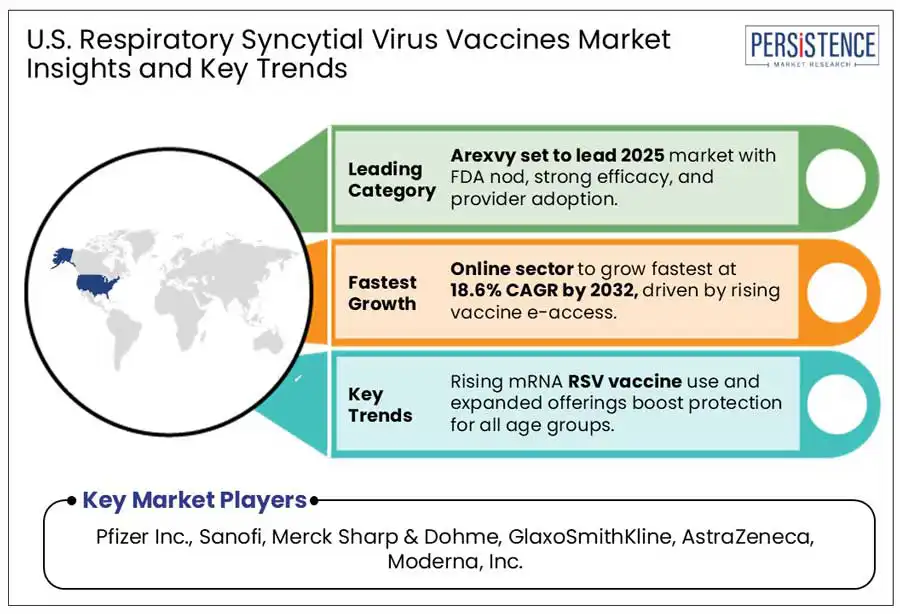
Advancements in online platforms, on the other hand, are also creating a lucrative outlook for the high sales of respiratory syncytial virus vaccines via online pharmacies because of ease in product delivery and attractive discount offers for customers. RSV-associated infections are one of the major causes of morbidity as well as mortality among neonates. Causes of such infections include the presence of chronic lung disease of prematurity, hemodynamically significant congenital heart disease, and preterm birth, which is less than 35 weeks of gestation stage.
The high cost of management of RSV is expected to be a restraint to the U.S. respiratory syncytial virus vaccines market growth. Rising cases of RSV is leading to an increase in the number of hospitalizations. Since there is no standard treatment for RSV, the cost for hospitalization increases and imposes monetary constraints on patients, including the high cost of Palivizumab, which has a larger availability and higher rate of adoption, owing to its efficacy.
Additionally, the price of the technology necessary for developing RSV therapies is high. This factor is expected to further hinder the growth of the RSV market in the U.S. Pharmaceutical companies have encountered major challenges in the development of vaccines for the prevention of RSV, which can be understood from the approval of only a single product, i.e. Palivizumab, in the past decade. A large amount of vaccine and monoclonal antibody candidates have failed to meet the needed efficacy and suffered severe hindrances in the late stage of development, which has limited the sales of respiratory syncytial virus vaccines.
Arexvy - RSVPreF3 OA (GSK3844766A) is expected to lead RSV vaccine sales during the forecast period in the U.S. GSK's Arexvy launched in 2023 demonstrated 94% efficacy against severe RSV illness. The drug is an adjuvanted vaccine which provides active immunization against lower respiratory tract disease (LRTD) caused by RSV in individuals aged 60 and above.
Further in 2024, the U.S FDA approved Arexvy for adults aged 50 – 59 at the increased risk of RSV. More than 9 million U.S. individuals have been administered with the shots of Arexvi drug since its approval contributing significantly to the growing market for respiratory syncytial virus vaccines in the U.S. Its widespread availability in major U.S. pharmacies and strong healthcare provider recognition have further contributed to its market dominance
Institutional sales lead among all distribution channels in the U.S. respiratory syncytial virus vaccines market and show a promising growth at a CAGR of 13.7% during the forecast period. This is due to increasing number of research intuitions in the U.S. where various research scientists are involved in the discovery of ResVax, Nirsevimab (MEDI8897), MVA-BN RSV, and other RSV vaccines.
In addition, the presence of leading research institutions in the country is contributing to the market growth, such as Harvard University, Stanford University, Massachusetts Institute of Technology (MIT), and others, where research related to vaccines and other drugs takes place.
Attributed to the above factors, institutional sales are displayed as the most preferred form of distribution channel for respiratory syncytial virus vaccines in the U.S. market.
Pfizer Inc., Sanofi, Merck Sharp & Dohme, GlaxoSmithKline, SOBI, Johnson & Johnson, Bavarian Nordic, Novavax, AstraZeneca, Moderna, Inc., Codagenix Inc., Intravacc BV., and Alphavax, Inc. are established manufacturers of respiratory syncytial virus vaccines.
All these companies are committed to strategically capitalizing on growth opportunities by advancing their own product pipelines, maximizing the value of existing products, and indulging in various business development activities. The U.S. respiratory syncytial virus vaccines market is further becoming more competitive with the FDA's approval of Moderna's mRESVIA, joining GSK's Arexvy and Pfizer's Abrysvo in the landscape.
The global market is set to reach US$ 604.8 Mn in 2025.
The market is projected to record a CAGR of 15.6% during the forecast period from 2025 to 2032.
Increasing awareness of RSV’s impact on vulnerable populations, growing vaccine adoption, and advancements in vaccine technology for both adults and infants is expected to drive the global market.
Institutional sales is the leading segment for distribution channel.
Pfizer Inc., Sanofi, Merck Sharp & Dohme, and GlaxoSmithKline are some leading players.
|
Report Attribute |
Details |
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis Units |
Value: US$ Mn/Bn Volume: Units |
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
|
Customization and Pricing |
Available upon request |
By Drug:
By Distribution Channel:
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author