ID: PMRREP5552| 189 Pages | 29 Sep 2025 | Format: PDF, Excel, PPT* | Healthcare

The global pap smear & hpv testing market is likely to be valued at US$7.5 Bn in 2025 and reach US$15.2 Bn by 2032, growing at a CAGR of 10.6% during the forecast period from 2025 to 2032.
The Pap Smear & HPV Testing market is experiencing robust growth driven by increasing awareness of cervical cancer screening, advancements in diagnostic technologies, and the rising prevalence of HPV-related diseases.
| Key Insights | Details |
|---|---|
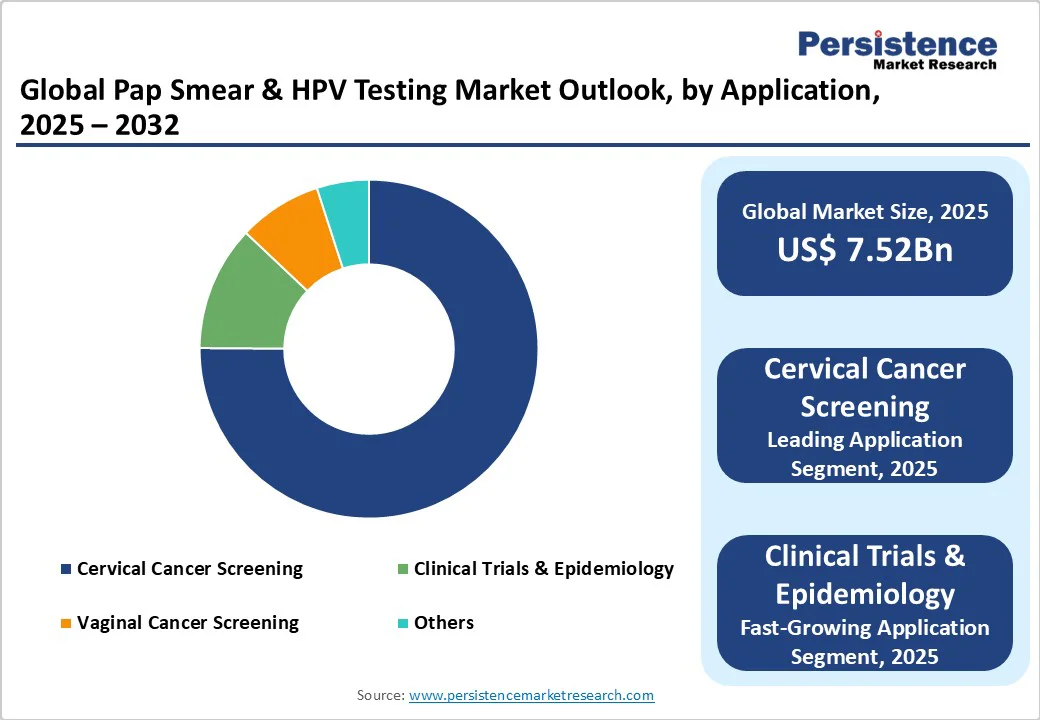
| Pap Smear & HPV Testing Market Size (2025E) | US$ 7.52 Bn |
| Market Value Forecast (2032F) | US$15.2 Bn |
| Projected Growth (CAGR 2025 to 2032) | 10.6% |
| Historical Market Growth (CAGR 2019 to 2024) | 9.8% |
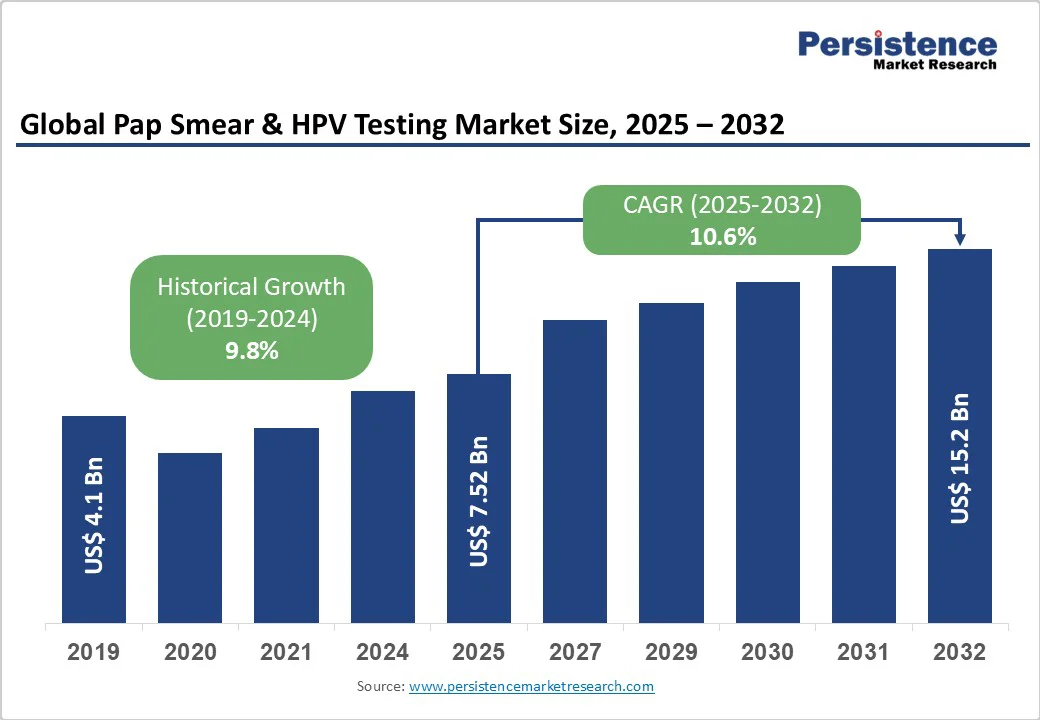
The Pap Smear & HPV Testing market is rapidly growing, driven by heightened global awareness of cervical cancer and expanding government screening programs. Cervical cancer remains a critical health challenge, with the World Health Organization estimating 660,000 new cases in 2024 alone. Early detection through Pap smear and HPV testing is vital for reducing mortality by identifying precancerous changes.
Governments worldwide are reinforcing screening efforts, such as the U.S. Preventive Services Task Force recommending routine HPV testing for women aged 30-65 and India’s Ayushman Bharat program, integrating cervical cancer screening into primary healthcare.
Industry leaders, such as Hologic, Inc., have reported increased sales of HPV testing kits in 2024, reflecting a rising demand. Innovations in high-sensitivity HPV tests, combined with robust public health campaigns promoting women’s health, are poised to sustain market momentum. These factors collectively ensure that the market will continue to expand steadily through 2032, thereby enhancing early detection and prevention worldwide.
The Pap smear & HPV testing market faces notable challenges, primarily due to the high costs of advanced diagnostic technologies and limited healthcare access in low-income regions. Leading HPV testing instruments from companies such as QIAGEN and Roche require significant financial investment, which many healthcare facilities in developing countries cannot afford.
In 2024, the average cost of an HPV test ranged between $30 and $100, creating a substantial barrier in budget-constrained areas such as Sub-Saharan Africa.
Furthermore, the scarcity of trained healthcare professionals and inadequate medical infrastructure in rural and underserved regions limit the effective adoption of both Pap smear and HPV testing. These challenges are compounded by competition from low-cost alternatives such as visual inspection with acetic acid (VIA), which, although less accurate, offer a more affordable screening option. Collectively, these factors restrict market penetration and overall growth potential in cost-sensitive markets, slowing progress toward widespread cervical cancer screening.
The integration of artificial intelligence (AI) with point-of-care (POC) testing is revolutionizing the market by enhancing diagnostic accuracy and accessibility. AI-driven platforms significantly enhance Pap smear analysis by minimizing human error and improving the detection of abnormal cells, resulting in earlier and more reliable diagnoses.
Leading companies, such as BD and Thermo Fisher Scientific, are pioneering AI-based software that automates slide analysis, thereby increasing efficiency and throughput in laboratories. Meanwhile, POC HPV testing devices from innovators such as NURX Inc. provide rapid, reliable results in remote or underserved areas, overcoming traditional accessibility barriers.
Government initiatives, including the EU’s Horizon 2020 program, are funding advancements in AI-driven diagnostics, fostering the development of cost-effective and scalable screening tools. These innovations are expected to drive market growth and improve cervical cancer screening outcomes globally through 2032, particularly in resource-limited settings.
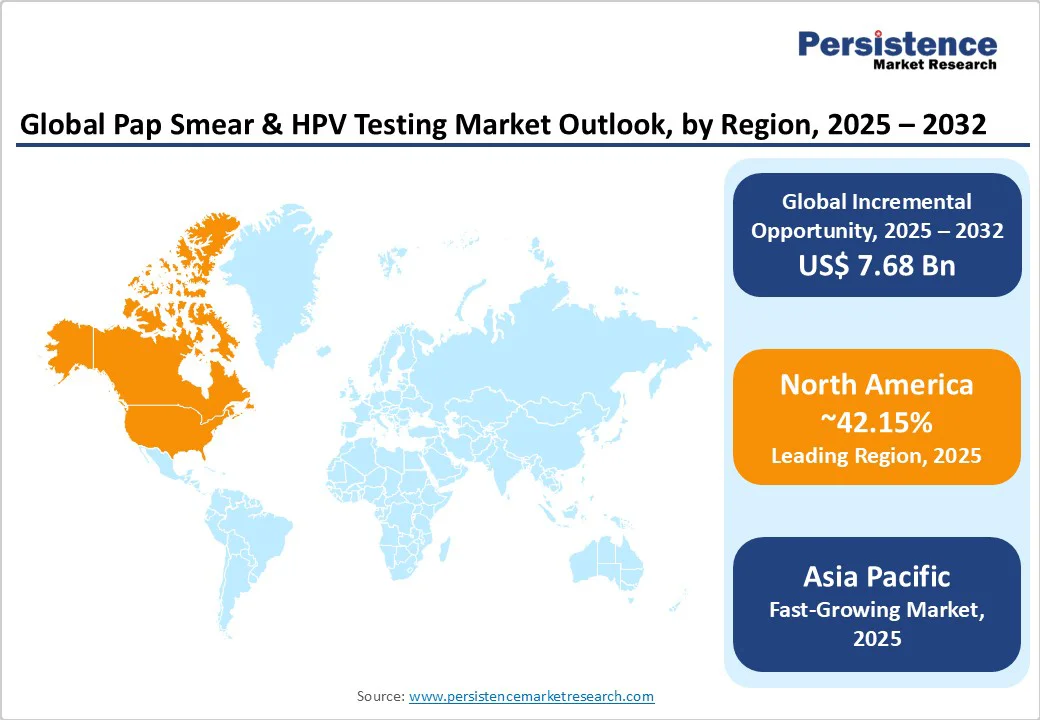
North America is projected to dominate the market, accounting for nearly 42.1% share in 2025. This leadership is fueled by the region’s advanced healthcare infrastructure, high cervical cancer screening rates, and a strong presence of key industry players, including Hologic, Inc., Quest Diagnostics, BD, and Abbott. These companies benefit from well-established distribution networks that supply hospitals, clinics, and diagnostic labs across the U.S. and Canada.
The U.S. healthcare system alone is expected to spend $5.7 trillion by 2026, according to the Centers for Medicare & Medicaid Services, supporting continued demand for screening technologies. In Canada, universal healthcare and national screening programs endorsed by organizations such as the Canadian Cancer Society promote routine Pap and HPV testing. Furthermore, growing consumer preference for high-sensitivity HPV tests, coupled with government funding for cancer prevention, bolsters the region’s dominance and drives innovation and accessibility in cervical cancer diagnostics.
Asia Pacific is the fastest-growing market for Pap smear and HPV testing, driven by its vast population base, rising healthcare investments, and increasing awareness of cervical cancer prevention. Countries such as China and India are spearheading regional growth through large-scale government screening initiatives.
China’s National Health Commission reported a screening coverage of 51.5% among women aged 35-64 in 2023-2024, significantly boosting demand for HPV testing kits. In India, the Ayushman Arogya Mandirs and National Health Mission (NHM) have screened over 10.18 crore (101.8 million) women aged 30-65 as of July 2025, underscoring strong public health engagement.
The region also benefits from an expanding diagnostic landscape, with local players such as Seegene Inc. and global giants like Roche and BD scaling up their operations. The growing prevalence of HPV-related diseases and robust government-led health campaigns further ensure that the Asia Pacific will remain a dominant and high-growth region in cervical cancer screening through 2032.
Europe stands as the second-fastest-growing region in the Pap smear and HPV testing market, driven by robust regulatory frameworks, rising awareness of cervical cancer prevention, and substantial investments in diagnostic innovation.
Countries such as Germany and the UK lead this momentum through well-established public health systems and organized screening programs. Germany’s robust diagnostic infrastructure and high adoption of molecular testing technologies support the expansion of HPV screening.
In the UK, the National Health Service (NHS) offers a nationwide cervical screening program, which encourages early detection and contributes to market growth. Leading diagnostic companies such as QIAGEN and Roche continue to innovate with advanced testing platforms and AI-driven tools.
Additionally, the EU’s Horizon 2025 program promotes cutting-edge research and development in digital and AI-enabled diagnostics, further enhancing demand for high-sensitivity and automated testing solutions. This combination of regulatory support, technological leadership, and public health initiatives positions Europe for sustained growth through 2032.
The global Pap Smear & HPV Testing market is highly competitive, characterized by a mix of established players and innovative newcomers. Companies such as Abbott, QIAGEN, BD, and Hologic, Inc. dominate through extensive product portfolios and global distribution networks.
The Pap Smear & HPV Testing market is fragmented, with regional players such as Seegene Inc. focusing on localized offerings in the Asia Pacific. Key players are investing in AI-driven diagnostics and point-of-care testing to enhance market share, driven by demand for high-sensitivity HPV tests and automated Pap smear analysis.
The Pap Smear & HPV Testing market is projected to reach US$7.52 Bn in 2025.
Rising awareness, government-led screening programs, and advancements in diagnostic technologies are the key market drivers.
The Pap Smear & HPV Testing market is poised to witness a CAGR of 10.6% from 2025 to 2032.
Advancements in AI-driven diagnostics and point-of-care testing are the key market opportunities.
Abbott, QIAGEN, BD, Hologic, Inc., and F. Hoffmann-La Roche Ltd are key market players.
| Report Attribute | Details |
|---|---|
| Historical Data/Actuals | 2019 - 2024 |
| Forecast Period | 2025 - 2032 |
| Market Analysis | Value: US$ Bn/Mn, Volume: As Applicable |
| Geographical Coverage |
|
| Segmental Coverage |
|
| Competitive Analysis |
|
| Report Highlights |
|
By Test Type
By Product & Service
By Application
By End-use
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author