ID: PMRREP35375| 170 Pages | 30 May 2025 | Format: PDF, Excel, PPT* | Healthcare

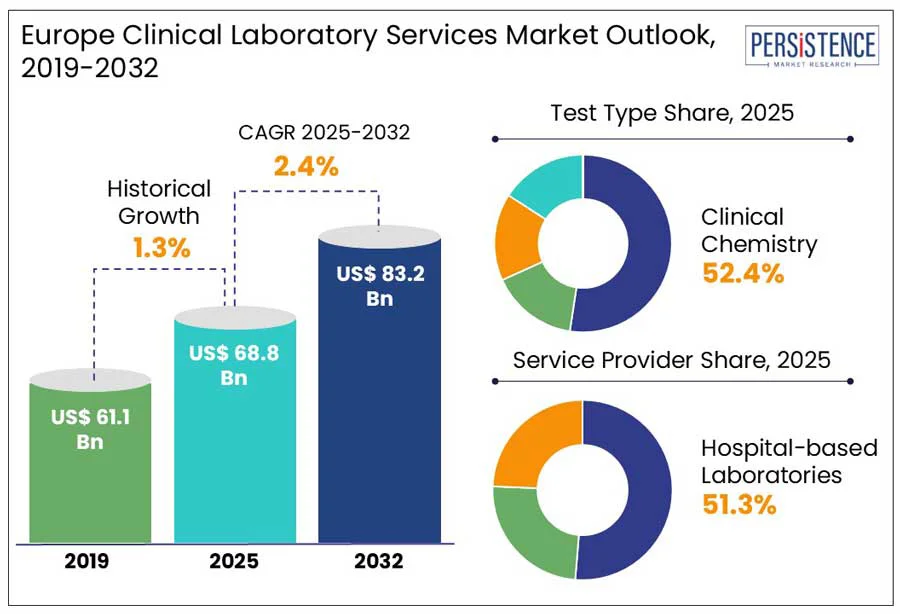
The Europe clinical laboratory services market size is predicted to reach US$ 83.2 Bn in 2032 from US$ 68.8 Bn in 2025. It will likely witness a CAGR of around 2.4% in the forecast period between 2025 and 2032.
Europe clinical laboratory services market is undergoing a fundamental transformation, reshaped by regional decentralization and data-driven diagnostics. No longer limited to routine blood work or urinalysis, laboratories are now central to early disease detection, pandemic preparedness, and precision medicine. From the cross-border pathology integration in the Nordics to the AI-backed molecular testing labs in Germany, the continent is setting new benchmarks for diagnostic excellence.
Key Industry Highlights
|
Market Attribute |
Key Insights |
|
Europe Clinical Laboratory Services Market Size (2025E) |
US$ 68.8 Bn |
|
Market Value Forecast (2032F) |
US$ 83.2 Bn |
|
Projected Growth (CAGR 2025 to 2032) |
2.4% |
|
Historical Market Growth (CAGR 2019 to 2024) |
1.3% |
Government initiatives are anticipated to significantly propel the Europe clinical laboratory services market growth in the foreseeable future, reveals Persistence Market Research. These are augmenting developments in research capabilities and diagnostic infrastructure. A notable development is the implementation of the European Health Data Space (EHDS), which came into force in 2025. This norm mandates EU member states to establish Health Data Access Bodies and digital health authorities.
It further enables secure cross-border access to health data and facilitates standardized electronic health records. By the end of 2031, the EHDS aims to make comprehensive health data, including imaging and laboratory results, accessible across the EU. Such initiatives are estimated to enhance patient care outcomes and improve collaborative research. The EU4Health program, implemented in 2021, also focuses on enhancing access to services and improving healthcare systems.
Potential errors in the clinical testing process are considered a significant bottleneck to enhancing trust and reliability in laboratory services across Europe. Pre-analytical errors such as patient identification mistakes, improper storage, or sample mislabeling account for around 60 to 70% of total laboratory errors, as per the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM).
Germany’s Proficiency Testing Programs also reported that more than 12% of participating labs in 2022 had at least one critical error in blood sample handling or registration. These issues are likely to require costly repeat testing, result in misinterpretations, or delay diagnosis, thereby burdening both patients and healthcare professionals.
Increasing partnerships between clinical laboratory service providers and universities are anticipated to create new opportunities in Europe through 2032. These partnerships are propelling innovation and extending access to novel diagnostics. A significant example is the Leiden Bio Science Park in the Netherlands, which integrates more than 215 organizations, including Leiden University Medical Center and Leiden University. This partnership focuses on biotechnology for biopharmaceutical and medical applications, including novel laboratory casework setups to support complex experimental workflows.
In the U.K., the Norwich Research Park exemplifies a successful collaboration between academia and clinical services. It brings together several research institutions, the Norfolk and Norwich University Hospital, and the University of East Anglia to spur innovations in environmental, health, and genomics research. Sweden's Science for Life Laboratory (SciLifeLab) further represents a key collaboration, involving Uppsala University, Karolinska Institutet, Stockholm University, and KTH Royal Institute of Technology. SciLifeLab serves as a national center for large-scale research in molecular biology, delivering expertise and innovative technologies to life scientists in Sweden.
Based on test type, the Europe clinical laboratory services market is trifurcated into human and tumor genetics, clinical chemistry, and medical microbiology and cytology. Among these, clinical chemistry tests are expected to generate a share of nearly 52.4% in 2025 and lead the market. This is attributed to their key role in monitoring and diagnosing a wide spectrum of acute and chronic conditions. These tests are required in the routine assessment of electrolyte balance, organ health, and metabolic functions, which makes them important in both primary care and hospital settings.
Human and tumor genetics testing, on the other hand, is predicted to witness a considerable CAGR from 2025 to 2032, owing to the expanding role of targeted therapies and personalized medicine. The European Medicines Agency (EMA), for example, approved more than 15 new personalized oncology drugs between 2022 and 2024. These require specific biomarker or genetic testing before prescription, thereby influencing demand for genomic diagnostics. BRCA1/2 mutation testing, for instance, is now routinely performed in ovarian and breast cancer cases across France and Germany, allowing for precise treatment with PARP inhibitors.
Based on service provider, the Europe clinical laboratory services market is divided into hospital-based laboratories, standalone laboratories, and clinic-based laboratories. Out of these, hospital-based laboratories are predicted to dominate by holding a share of approximately 51.3% in 2025. It is mainly due to the rising shift toward integrated diagnostics in hospital ecosystems, mainly for complex and acute care. Hospitals are under constant pressure to lower diagnostic turnaround times for inpatients and emergency cases, boosting investments in in-house laboratory capabilities, including infectious disease testing and oncology.
Clinic-based laboratories, on the other hand, witness steady growth, backed by the surge of community-based and decentralized healthcare models. These models emphasize localized, and quick diagnostic services for outpatient care. As healthcare systems across Europe strive to improve early detection and lower the burden on hospitals, clinics are being empowered with affiliated or in-house laboratories supported by efficient laboratory management services. In Spain, for example, Madrid and Catalonia, have extended diagnostic capabilities across primary health centers, as per Spain’s Ministry of Health.
The U.K. will likely rise at a steady pace owing to evolving healthcare demands, technological innovations, and various public-private collaborations. It is projected to account for a share of about 34.7% in 2025. A key trend in the country is the rising dependence on private firms to supplement diagnostic services. The National Health Service (NHS), for instance, allocated a record £216 Mn in 2024 to private firms for the interpretation of X-rays and scans. Such outsourcing, while addressing immediate backlogs and catering to urgent care, is predicted to raise concerns about the long-term sustainability of NHS radiology services.
The private sector’s role is further extending as patients’ demand alternatives to the NHS, on the back of long waiting times. Private healthcare providers are experiencing high demand, with a few performing a significant number of procedures funded by the NHS. This shift points toward a rising interdependence between private and public healthcare services in the U.K. Laboratory automation is also gaining impetus as key companies are launching innovative automated solutions to improve efficiency.
France is witnessing a surging adoption of Laboratory Developed Tests (LDTs), specifically among private labs as they enable rapid deployment of specialized diagnostics. A key trend in the country is the consolidation of private laboratories. About six prominent groups controlled more than two-thirds of city-based laboratories, as of 2023 and it exhibited a sharp drop from nearly 5,000 independent labs in 2008 to around 400 today. This consolidation has pushed investments in innovative diagnostics and enabled economies of scale.
Government initiatives such as the Ma Santé 2022 plan are playing significant roles in France. The plan focuses on reorganizing healthcare delivery and improving digital health infrastructure. Similar policies are implemented to promote preventive healthcare measures and support the development of modern diagnostic facilities. These are further anticipated to extend the use of clinical laboratory services.
Germany is currently witnessing decent growth in the field of clinical laboratory services. This is primarily due to a rising emphasis on research and development, market consolidation, and the emergence of new technologies. Renowned companies such as SYNLAB Group, headquartered in Munich, are dominating the market with a wide range of services and extensive networks. SYNLAB, for instance, currently operates about 500 laboratories and performed 600 Mn tests in 2022 alone, achieving sales revenue of nearly €3.25 Bn.
Technological developments are further transforming the landscape, with initiatives such as the EMPAIA project promoting AI-assisted digital pathology. EMPAIA has integrated 14 AI-based image analysis applications from eight vendors, showcasing the potential for standardized AI integration in clinical settings. Additionally, laboratory informatics is playing an important role in enhancing real-time decision-making, data accuracy, and workflow efficiency. The country, however, faces challenges such as a shortage of skilled professionals, making it difficult to recruit medical-technical assistants and lab physicians.
The Europe clinical laboratory services market is consolidated in nature. Large-scale multinational diagnostic firms are leading the market with strategic acquisitions and broad testing networks that continue to absorb small-scale players. This consolidation is mainly propelled by the demand for cost-efficiency, standardization, and integration of novel diagnostic platforms across national borders.
Public-private collaborations are also gaining momentum, specifically in the Netherlands, France, and Germany. Government agencies in these countries are emphasizing the optimization of healthcare spending. Hence, they are outsourcing routine testing to private labs, further creating opportunities for companies to demonstrate their automation, scale, and quality compliance.
The Europe clinical laboratory services market is projected to reach US$ 68.8 Bn in 2025.
Increasing collaborations between universities and private labs and the emergence of automation are the key market drivers.
The Europe clinical laboratory services market is poised to witness a CAGR of 2.4% from 2025 to 2032.
Rising shift toward outsourcing and increasing demand for cost-effective testing services are the key market opportunities.
Eurofins Scientific, UNILABS, and SYNLAB International GmbH are a few key market players.
|
Report Attribute |
Details |
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis Units |
Value: US$ Bn/Mn, Volume: As Applicable |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
|
Customization and Pricing |
Available upon request |
By Test Type
By Service Provider
By Application
By Country
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author