ID: PMRREP35451| 164 Pages | 26 Jun 2025 | Format: PDF, Excel, PPT* | Healthcare

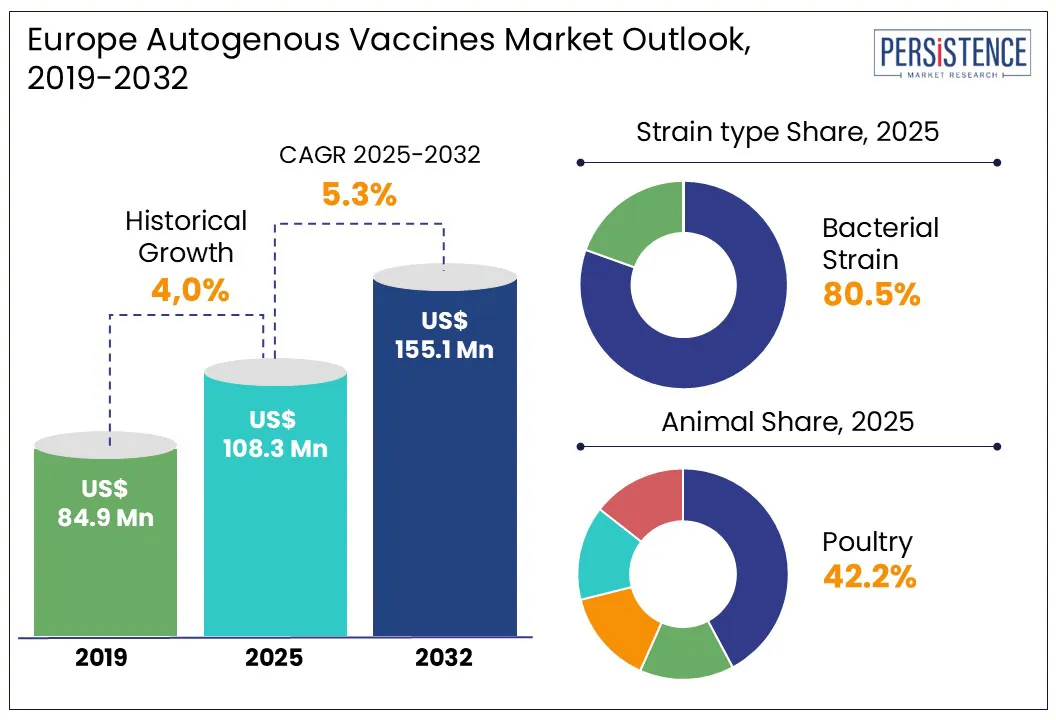
According to Persistence Market Research, Europe autogenous vaccines market size is likely to be valued at US$ 108.3 Mn in 2025 and reach US$ 155.1 Mn by 2032, growing at a CAGR of 5.3% during the forecast period from 2025 to 2032.
Europe autogenous vaccines market is poised for significant growth, driven by strong regional emphasis on preventive veterinary care, antimicrobial stewardship, and tailored disease management strategies. France, for instance, launched its second commercial vaccination campaign against Avian Influenza H5N1 in October 2024, following the success of the inaugural campaign that vaccinated over 50 million ducks.
Additionally, France increased the number of bluetongue vaccines to be given for free to farmers to 11.7 million doses in 2024, up from 6.4 million doses previously, in response to outbreaks affecting cattle and sheep. These initiatives underscore the growing emphasis on preventive measures and tailored vaccination strategies in the region.
Furthermore, ANSES, the French Agency for Food, Environmental and Occupational Health & Safety, reinforced its 2025 agenda to support emerging disease monitoring and vaccine research. France’s collaboration with the African Union and Gavi also highlights Europe’s role in global vaccine equity and manufacturing expansion. These developments reinforce the growing reliance on customized, farm-specific vaccines to improve animal health outcomes.
Key Industry Highlights:
|
Europe Market Attribute |
Key Insights |
|
Autogenous Vaccines Market Size (2025E) |
US$ 108.3 Mn |
|
Market Value Forecast (2032F) |
US$ 155.1 Mn |
|
Projected Growth (CAGR 2025 to 2032) |
5.3% |
|
Historical Market Growth (CAGR 2019 to 2024) |
4.0% |
Europe autogenous vaccines market is being driven by a growing need for targeted, farm-specific disease prevention amid rising concerns over antimicrobial resistance (AMR). With the European Union’s ban on the preventive use of antibiotics in animal feed (Regulation EU 2019/6) effective since January 2022, producers are turning to autogenous vaccines as a sustainable alternative. These vaccines offer tailored protection against pathogens not covered by commercial products, helping to enhance herd immunity and reduce reliance on broad-spectrum antimicrobials.
Regulatory support and declining antimicrobial sales down 53% between 2011 and 2022 in Europe (European Medicines Agency (EMA), 2023) are reinforcing this trend. Disease outbreaks such as bluetongue virus and epizootic haemorrhagic disease have further emphasized the importance of proactive immunization. Advances in vaccine development, including faster antigen production and approval pathways, also support market growth. As awareness among farmers and veterinarians increases, autogenous vaccines are emerging as essential tools in precision herd health management across Europe.
A key restraint in the European autogenous vaccines market is the limited understanding of veterinary disease epidemiology, particularly for region-specific and emerging pathogens. Diseases such as virulent Newcastle Disease in poultry highlight significant knowledge gaps in how pathogens evolve and spread, due to rapid genetic mutations and inadequate surveillance. These gaps hinder the development of effective, tailored vaccines.
Additionally, the complex genetic diversity of pathogens across Europe and limited insight into antigen-immunogenic structures make it difficult to identify appropriate vaccine targets. This challenge is compounded by the lack of collaboration between veterinary research institutes and vaccine manufacturers, resulting in poor knowledge of immune responses, protective mechanisms, and clinical outcomes. The absence of robust data-sharing systems further delays the creation of herd-specific formulations. To unlock the full potential of autogenous vaccines in Europe, coordinated efforts in research, diagnostics, and industry partnerships are essential to enhance precision, speed, and efficacy in vaccine development.
The European autogenous vaccines market presents strong growth opportunities driven by rising awareness among farmers and veterinarians regarding targeted disease prevention and herd-specific health management. With growing concerns over antimicrobial resistance (AMR), especially in livestock production, there is a clear shift toward safer and more sustainable alternatives. Autogenous vaccines, developed from pathogens isolated within individual herds or flocks, offer tailored protection with reduced impact on microbiota and lower risk of resistance.
In November 2024, Ceva Animal Health announced a €75 million investment to build a 7,000 m² vaccine manufacturing facility in Hungary. Scheduled to open by the end of 2026, this plant is expected to produce fermentation-based multicomponent inactivated vaccines, with a capacity of over 8 billion doses annually. This expansion is anticipated to strengthen Ceva’s commitment to preventive medicine and tailored vaccine solutions for emerging diseases, supporting the growing demand for autogenous vaccines across Europe.
Additionally, veterinary outreach programs, diagnostic advancements, and EU initiatives such as the Animal Health Law (Regulation 2016/429) are promoting responsible disease control practices. Central European countries such as the Czech Republic, Hungary, and Slovakia, where autogenous vaccine use is well established, continue to drive adoption. As knowledge-sharing platforms expand and farmers increase biosecurity efforts, demand for customized vaccination strategies is expected to grow significantly.
Bacterial strain segment is projected to hold a revenue share of over 80% in 2025 within the Europe autogenous vaccines market. This dominance is driven by the high prevalence of bacterial infections in livestock, such as swine dysentery, salmonellosis, and E. coli-related diseases, which are often specific to individual herds or farms.
Autogenous vaccines using bacterial strains offer a targeted approach, enabling veterinarians to develop precise formulations based on locally circulating pathogens. These vaccines are particularly valuable when commercial options are unavailable or ineffective against farm-specific bacterial variants. Additionally, the rise in antimicrobial resistance has prompted regulatory bodies and producers to favor non-antibiotic solutions, further boosting demand for customized bacterial vaccines as a proactive disease management strategy.
Poultry segment is expected to dominate the animal category in 2025 with nearly 42.2% of Europe autogenous vaccines market share. This is due to the high density of poultry farming across Europe and the frequent occurrence of herd-specific diseases such as Newcastle disease and infectious bronchitis, which require customized vaccination solutions. Compared to swine, fish, horse, and other animals, poultry production is more widespread, with rapid turnover and significant economic impact, driving greater vaccine demand.
Swine, however, is the most lucrative sector with a CAGR of 5.9% over the forecast period due to increasing concerns about diseases like influenza A virus and porcine reproductive and respiratory syndrome (PRRS). Swine farms often face complex, evolving pathogens, creating strong demand for tailored autogenous vaccines to improve herd health and productivity.
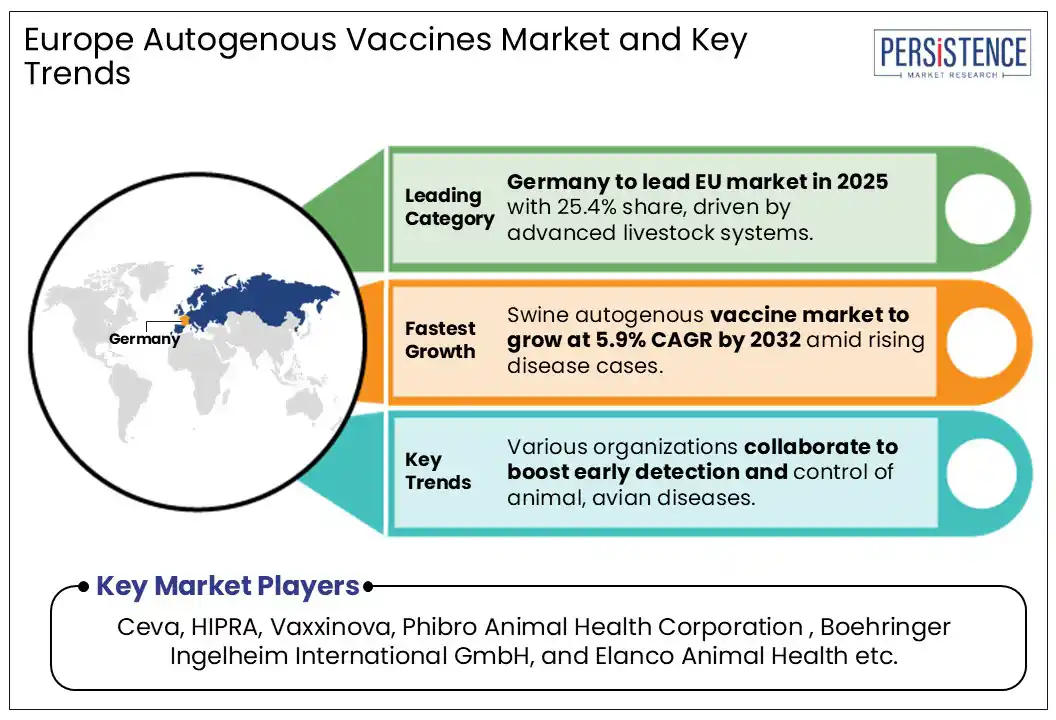
Germany is anticipated to hold 25.4% of the Europe market share in 2025 due to its large and well-established livestock industry, especially in swine and poultry farming. The country’s strong focus on advanced animal health management, rigorous regulatory framework, and early adoption of innovative veterinary solutions support market growth. For instance, in February 2025, the Dopharma Group and its subsidiary Ripac-Labor GmbH officially broke ground on a cutting-edge autogenous vaccine production facility in Potsdam, Germany, marking a major expansion initiative.
Germany’s commitment to combating antimicrobial resistance through stringent regulations encourages the use of autogenous vaccines as sustainable alternatives. Additionally, robust veterinary infrastructure, active research collaborations, and increasing farmer awareness about targeted disease prevention further drive demand for customized vaccines in the region.
The UK is estimated to be the most lucrative market over the forecast period, with a projected CAGR of 6.1% between 2025 and 2032, driven by its advanced livestock sector and strong emphasis on sustainable farming practices. Increasing concerns over AMR and government initiatives promoting responsible antibiotic use are accelerating demand for alternative disease prevention methods such as autogenous vaccines.
Additionally, the UK’s well-developed veterinary services, growing adoption of precision livestock farming, and rising awareness among farmers about herd-specific health management contribute significantly to market growth. Ongoing investments in research and development further support innovation and the adoption of customized vaccines across various livestock and aquaculture sectors.
In February 2025, Mowi Scotland reported a 35% reduction in overall biomass mortality across all seawater farms in 2024, attributed to enhanced biosecurity, improved vaccination programs-including autogenous vaccines developed in collaboration with Ridgeway Biologicals-and favourable environmental conditions. This success highlights the increasing role of autogenous vaccines in improving animal health outcomes in the UK, reinforcing their potential as a key tool in sustainable animal disease management.
Key players in the Europe autogenous vaccines market are focusing on adhering to evolving regulatory standards to facilitate market access and strengthen veterinarian trust. Manufacturers are boosting investments in research and development to improve strain identification, shorten production cycles, and create precision-targeted vaccines designed for specific farm pathogens and emerging regional challenges.
The Europe autogenous market size is set to reach US$ 108.3 Mn in 2025.
The market is projected to record a CAGR of 5.3% during the forecast period from 2025 to 2032.
Growing demand for tailored autogenous vaccines in aquaculture and livestock, decline in antimicrobial use, and increasing focus on preventative measures for herd health are the key driving factors for the market growth.
Ceva, HIPRA, Vaxxinova, Phibro Animal Health Corporation, Boehringer Ingelheim International GmbH, and Elanco Animal Health are a few leading players.
Germany is projected to dominate the European market in 2025.
|
Report Attribute |
Details |
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis Units |
Value: US$ Mn/Bn |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
|
Customization and Pricing |
|
By Strain Type
By Animal
By End-use
By Country
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author