ID: PMRREP28264| 220 Pages | 13 Oct 2025 | Format: PDF, Excel, PPT* | Healthcare

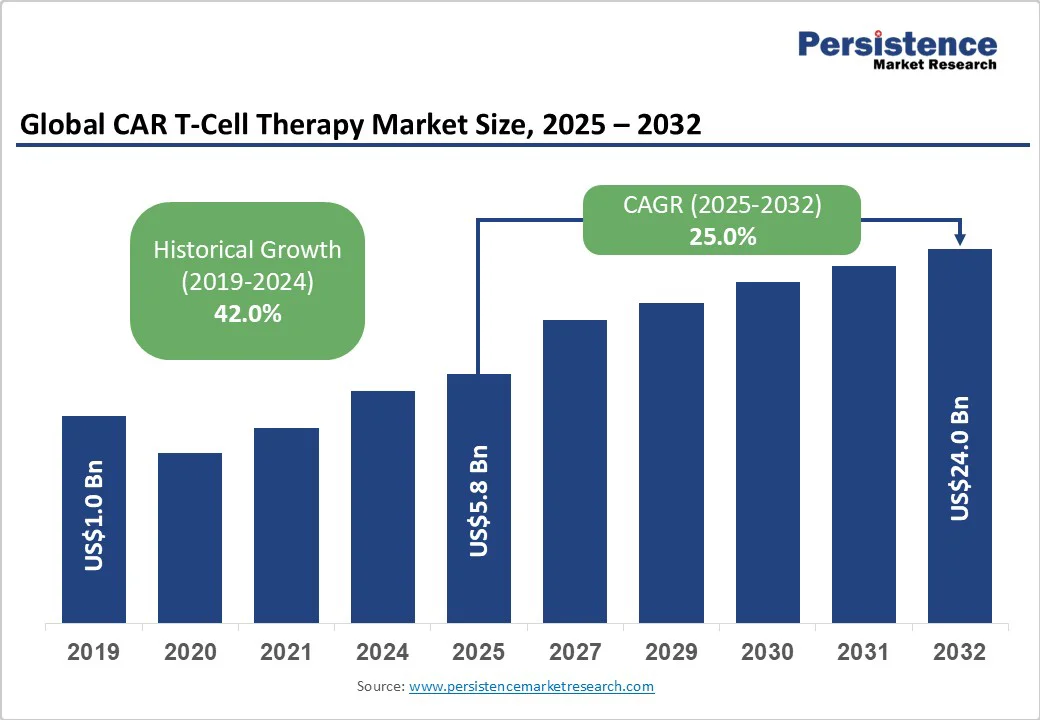
The global CAR T-cell therapy market size is likely to be valued at US$5.8 Billion in 2025, and is estimated to reach US$24 Billion by 2032, growing at a CAGR of 25% during the forecast period 2025−2032, driven by the increasing prevalence of hematological malignancies and expanding indications in oncology.
Advances in gene-editing technologies, supportive regulatory frameworks, and rising healthcare investments are enabling broader clinical access and expedited product development. These factors have created a robust growth environment underscored by increasing clinical approvals and expanding patient populations requiring personalized immunotherapies.
| Key Insights | Details |
|---|---|
|
CAR T-Cell Therapy Market Size (2025E) |
US$5.8 Bn |
|
Market Value Forecast (2032F) |
US$24 Bn |
|
Projected Growth (CAGR 2025 to 2032) |
25% |
|
Historical Market Growth (CAGR 2019 to 2024) |
42% |
Globally, cancer prevalence is rising, particularly hematologic malignancies such as non-Hodgkin lymphoma (NHL), acute lymphoblastic leukemia, and multiple myeloma, key targets for CAR T-cell therapies. In 2022, the International Agency for Research on Cancer reported 553,389 NHL cases worldwide, with a mortality rate of about 50%, reflecting a growing patient population in need of advanced oncological treatments. The American Cancer Society estimates over 80,000 new lymphoma diagnoses annually in the U.S., further driving demand for innovative therapies. This increasing disease burden, alongside the limited effectiveness of conventional treatments, is accelerating clinical adoption and commercial growth of CAR T-cell therapies.
Advancements in CAR T-cell technology, such as novel antigen targeting, integrated safety switches, and improved manufacturing processes, are boosting efficacy and safety, enhancing clinician confidence and patient access. Emerging innovations, including gene-editing tools such as CRISPR and the development of allogeneic (off-the-shelf) CAR T-cell products, promise to reduce production time and costs, enabling broader scalability. Regulatory bodies such as the U.S. FDA and European Medicines Agency have established accelerated approval pathways, signaling strong confidence in these therapies. This regulatory support, combined with technological progress, is fostering pipeline diversification and investment, positioning CAR T-cell therapy as a transformative approach in oncology.
The CAR T-cell therapy market faces significant challenges due to its high treatment costs, often exceeding US$400,000 per patient, creating substantial reimbursement and access barriers worldwide. Many healthcare systems, especially in emerging markets, lack adequate reimbursement frameworks to cover the extensive expenses of therapy production, administration, and post-treatment care. A National Health Service (U.K.) study highlights growing scrutiny of cost-effectiveness thresholds for innovative treatments, placing economic pressure on CAR T-cell therapies. These financial constraints limit widespread adoption, particularly in lower-income regions, restricting market penetration and slowing revenue growth. To address this, companies and policymakers are exploring innovative payment models, such as outcomes-based reimbursement, to reduce financial risks and improve accessibility.
Additionally, CAR T-cell therapies involve highly specialized, autologous manufacturing processes that require patient-specific cell harvesting, engineering, and reinfusion. This complexity, combined with limited manufacturing sites, specialized logistics, and strict quality controls, creates bottlenecks in timely delivery. COVID-19 disruptions exposed supply chain vulnerabilities, increasing delays and operational costs. Many manufacturers report capacity and scalability issues, impacting their ability to meet growing global demand. These supply constraints result in shortages and longer patient wait times, thereby creating commercial challenges.
The embedding of artificial intelligence (AI), machine learning (ML), and advanced biomarker discovery into CAR T-cell development is opening new frontiers to improve therapy personalization, efficacy, and safety. Predictive analytics are helping identify optimal patient candidates and anticipate adverse events, thereby improving clinical outcomes and reducing costs. Furthermore, evolving gene-editing platforms are facilitating the engineering of CAR T-cells with dual targeting capabilities and reduced immunogenicity. These advancements could substantially increase the addressable patient population by enabling therapy use in solid tumors and resistant cancer forms, potentially adding multiple billion dollars to the market by 2032. Industry collaboration with biotech startups and academic institutions focused on these convergent technologies is pivotal to capturing this emerging high-growth segment.
Capitalizing on unmet oncological needs of an expanding patient pool in the emerging economies of Asia Pacific, Latin America, and the Middle East is a high-value business avenue for market players. For instance, projections from the IARC show that there will be around 5.1 million cancer cases in China and 1.5 million in India in 2025. These, along with other developing economies of the region, are witnessing rising governmental healthcare expenditures. Enhancing regulatory frameworks and increasing local reimbursement coverage in these regions are facilitating clinical trial approvals and therapy accessibility, producing unprecedented opportunities in the years to come.
Currently, in 2025, Yescarta, marketed by Gilead Sciences via its Kite Pharma subsidiary, is the leading drug type segment in the market, capturing an estimated market share of approximately 35%. This leadership position is underpinned by Yescarta's early regulatory approvals across the U.S. and Europe, alongside robust clinical efficacy in treating relapsed or refractory large B-cell lymphoma and other hematologic malignancies. Its commercial success is further supported by a well-established distribution network, payer reimbursement acceptance, and ongoing label expansions. Yescarta’s strong presence in key oncology treatment centers globally has made it a preferred option for clinicians and patients requiring targeted immunotherapy, thereby driving significant revenue generation.
The highest CAGR is likely to be registered by Abecma, being the first FDA-approved CAR T-cell therapy targeting multiple myeloma, a rapidly growing oncology indication with increasing incidence globally. Abecma's robust clinical data demonstrating significant efficacy and an expanding label for multiple myeloma treatment in both the U.S. and international markets are key growth drivers. The rising prevalence of multiple myeloma, especially in aging populations, along with ongoing clinical trials for Abecma’s use in earlier disease stages and combinations with other therapies, contributes to this rapid uptake. Regulatory advances and increased reimbursement support have further accelerated Abecma's adoption.
CD19-targeted CAR T-cell therapies will continue to dominate the market with an estimated 2025 revenue share of approximately 55%, principally due to their efficacy in treating B-cell malignancies such as non-Hodgkin lymphoma and acute lymphoblastic leukemia. These therapies benefit from robust and extensive clinical data supporting durable remission rates in relapsed or refractory cases where conventional treatments often fail, reinforcing broad regulatory approvals across multiple geographies. The established safety profile and expanding indication labels further solidify their clinical adoption. Moreover, CD19 therapies have been integrated into many oncology treatment guidelines, enabling widespread clinical use in specialized cancer centers.
The BCMA (B-cell maturation antigen)-targeted CAR T-cell therapy segment is poised as the fastest-growing, driven by the rising global incidence of multiple myeloma, a disease primarily affecting plasma cells that exhibit high BCMA expression, making it an optimal target for CAR T-cell immunotherapies. BCMA-targeted therapies such as Abecma (idecabtagene vicleucel) and Carvykti (ciltacabtagene autoleucel) have demonstrated compelling clinical efficacy, characterized by high response rates and durable remissions in relapsed or refractory multiple myeloma patients. Regulatory approvals granted by the FDA and EMA have further catalyzed adoption, encouraging rapid market penetration.
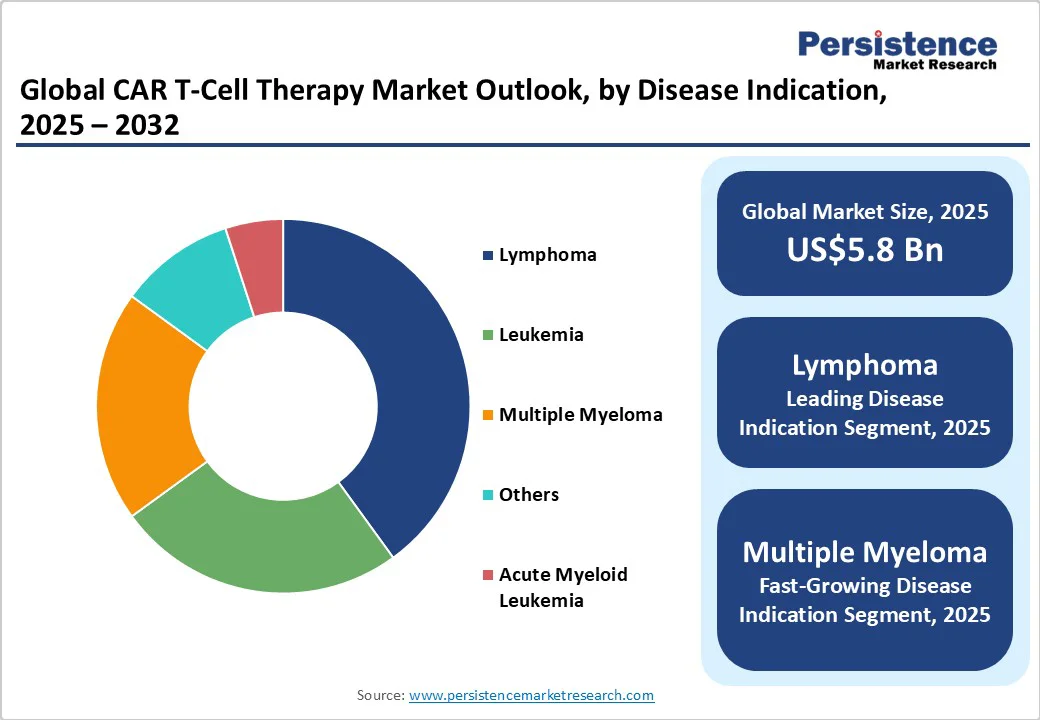
Lymphoma is predicted to hold the largest revenue share at an estimated 40% in 2025. The dominance of the segment is sustained by its high global prevalence and the well-established efficacy of CAR T therapies targeting aggressive and relapsed/refractory forms such as diffuse large B-cell lymphoma (DLBCL). The durable remission rates demonstrated in pivotal trials and inclusion in global treatment guidelines reinforce widespread clinical adoption. Factors such as increasing incidence of lymphoma subtypes, improved diagnostic capabilities, and expanded healthcare coverage in key markets are bolstering demand.
Multiple myeloma is the fastest expanding indication, driven by rising disease incidence, particularly in aging populations, with the U.S. estimating over 36,000 new cases in 2025 alone and a global market for multiple myeloma treatments projected to reach nearly US$42 Billion by 2032. Enhanced survival rates due to novel therapeutics, including CAR T-cell and bispecific antibodies, are transforming multiple myeloma into a more chronic condition, increasing patient pools eligible for advanced therapies. Ongoing approvals and clinical trials targeting earlier treatment lines and combinational regimens are expanding therapeutic applications.
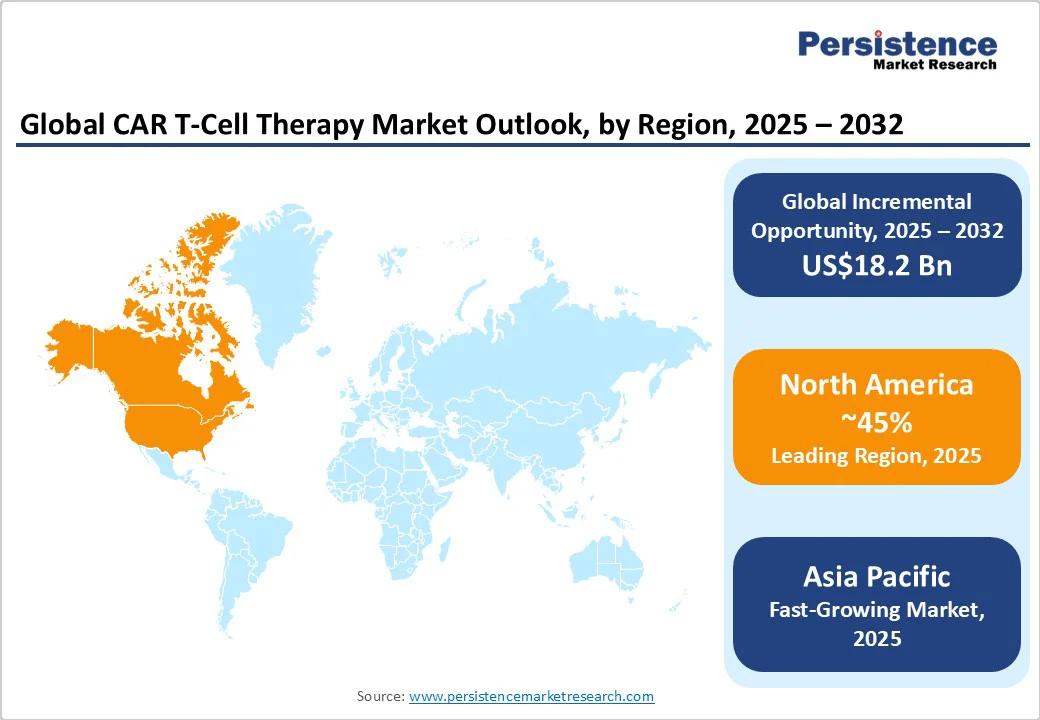
North America is projected to hold an approximate 45% market share in 2025, driven predominantly by the U.S., which commands high healthcare expenditure and an established advanced therapy ecosystem. The regional market benefits from a well-developed innovation landscape with numerous biotechnology hubs, strong venture capital funding, and a comprehensive regulatory framework provided by the FDA that supports accelerated approvals for novel therapies. Key factors fueling market growth include high disease awareness, early adoption of CAR T-cell therapies, and favorable reimbursement policies for cancer treatments.
Competitive intensity is marked by several major biopharma companies headquartered in the U.S., leveraging technological advances to expand product portfolios and enter new therapeutic indications. Investment trends spotlight increasing government and private sector funding for cell and gene therapy manufacturing facilities and R&D centers, enabling scalable production and clinical pipeline expansion. Regulatory evolution, facilitating breakthrough therapy designations and expanded access programs, further enhances patient uptake and market penetration.
Europe is expected to capture approximately 30% of the global market by 2025, with Germany, the U.K., France, and Spain being the key contributors. Regulatory harmonization under the EMA, alongside initiatives such as the European Advanced Therapy Medicinal Products (ATMPs) framework, is facilitating streamlined clinical trials and market entry.
The regional market is accelerating due to increasing oncology incidence, government-backed healthcare financing reforms, and growing clinical infrastructure dedicated to cell therapies. Germany leads in investment with strong reimbursement policies for CAR T-cell therapies, supporting adoption. Market growth is further propelled by expanding clinical trial activities and collaborations between academia and industry to advance therapy development. However, varying national regulatory processes and reimbursement landscapes require tailored market access strategies.
Asia Pacific is anticipated to emerge as the fastest-growing regional market. Key growth markets include China, Japan, India, and the ASEAN countries, where rising cancer prevalence, expanding healthcare infrastructure, and increasing government healthcare spending are driving demand. Regulatory agencies in China and Japan are adapting more flexible approval processes to accelerate access to innovative therapies.
Manufacturing advantages, including lower labor and production costs combined with growing biopharmaceutical investments, are attracting global and local companies to expand their presence. Strategic partnerships are enhancing clinical research capabilities, and domestic players are emerging with pipeline candidates tailored for regional patient populations. Even though variable reimbursement systems and market access challenges necessitate adaptive pricing models, the large patient populations and improving infrastructure of the region underscore its strategic importance for business expansion.
The global CAR T-cell therapy market structure is moderately consolidated, with leading companies collectively holding an estimated 70% market share as of 2025. Dominant players include Gilead Sciences (via Kite Pharma), Novartis AG, and Bristol-Myers Squibb, each leveraging established CAR T-cell products and diversified pipelines.
Market positioning is characterized by differentiation through product portfolios, strategic partnerships, and geographical presence. Smaller biotechs and emerging firms contribute innovation but have limited commercial scale. Continued consolidation through mergers and acquisitions is anticipated as companies seek technological synergies and expanded market footprints.
The CAR T-cell therapy market is projected to reach US$5.8 Billion in 2025.
The increasing prevalence of hematological malignancies, such as lymphoma, and expanding indications in oncology are driving the market.
The CAR T-cell therapy market is poised to witness a CAGR of 25% from 2025 to 2032.
Advances in gene-editing technologies, supportive regulatory frameworks, and the rise in clinical approvals for novel cancer therapies are key market opportunities.
Gilead Sciences, Inc., Novartis AG, and Bristol-Myers Squibb Company are some of the key players in the CAR T-cell therapy market.
| Report Attribute | Details |
|---|---|
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis |
Value: US$ Bn |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
By Drug Type
By Target Antigen
By Disease Indication
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author