ID: PMRREP35126| 194 Pages | 17 Jul 2025 | Format: PDF, Excel, PPT* | Healthcare

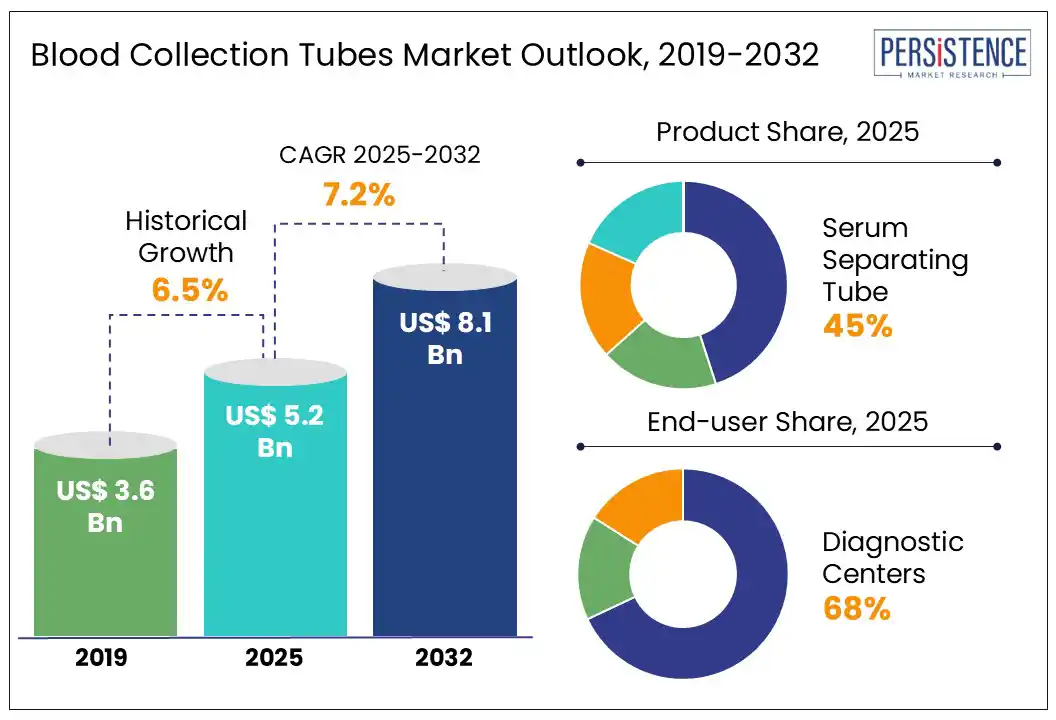
The global blood collection tubes market size is likely to be valued at US$ 5.2 Bn in 2025 and is estimated to reach US$ 8.1 Bn in 2032, growing at a CAGR of 7.2% during the forecast period 2025 – 2032.
Blood examination plays a vital role in disease screening, and selecting the appropriate blood collection tube is essential to ensure accurate results. These tubes are made of glass or plastic and are pre-filled with a vacuum, which determines the exact volume of blood to be drawn, ensuring consistency and accuracy in testing.
The blood collection tubes market growth is driven by the increasing prevalence of chronic diseases, advancements in diagnostic technologies, investments in healthcare infrastructure, and the rising demand for point-of-care testing and home healthcare services.
Blood collection tubes have color-coded caps, indicating the type of additive inside and its intended use. These tubes offer safety and convenience, and also minimize hemolysis and contamination. They allow multiple samples to be collected from one venipuncture using a multi-sample needle, thereby improving patient comfort.
Key Industry Highlights
|
Global Market Attribute |
Key Insights |
|
Blood Collection Tubes Market Size (2025E) |
US$ 5.20 Bn |
|
Market Value Forecast (2032F) |
US$ 8.1 Bn |
|
Projected Growth (CAGR 2025 to 2032) |
7.2% |
|
Historical Market Growth (CAGR 2019 to 2024) |
6.5% |
The increasing prevalence of chronic diseases such as diabetes, cardiovascular conditions, and cancer is a significant driver. These conditions require frequent blood tests for diagnosis, monitoring, and treatment, thereby increasing the demand for reliable and efficient blood collection solutions. For instance, in the U.S., approximately 38 million adults were diagnosed with diabetes in 2022, highlighting the need for consistent blood glucose monitoring. Diabetes mellitus requires serum for tests such as glucose, lipid profiles, and renal function to monitor disease management.
Cardiovascular diseases also account for nearly 31% of global deaths, highlighting the importance of regular blood tests in managing these conditions. As per WHO 2024, chronic diseases cause approximately 43 million deaths annually, accounting for about 75% of all global deaths. The availability of home collection services by laboratories has also increased the rate of blood testing. The increasing diagnosis of hereditary bleeding disorders, such as hemophilia A, hemophilia B, and von Willebrand disease, also requires blood tests and blood transfusions, which have surged the requirement for blood collection tubes.
Stringent regulatory requirements act as a restraint, primarily due to the increased time, cost, and complexity they impose. Manufacturers must comply with comprehensive standards such as FDA 510(k) clearance, EU CE marking under MDR/IVDR, ISO?13485 quality systems, Good Manufacturing Practices (cGMP), and extensive post-market surveillance. These regulations necessitate substantial investments in documentation, quality control, clinical testing, and ongoing compliance activities. Developing each new tube requires revalidation and regulatory filings for purity and biocompatibility, further delaying product launches and increasing the risk for non-compliance penalties.
For small and mid-sized manufacturers, these barriers can be burdensome. The financial and resource demands to establish and maintain certified quality management systems, manage change control, and conduct rigorous testing can inhibit innovation and market entry. The diversity of regulatory frameworks such as FDA, EMA, SAHPRA, ISO, and CDSCO add further operational complexity. These obligations create lengthy approval timelines, high costs for documentation, testing, and quality systems, and complex global coordination, slowing down product launches, limiting innovation, and raising barriers for smaller companies.
Advancements in materials have replaced traditional glass tubes with biocompatible plastics such as PET, which are shatterproof and lightweight. The development of silicone-coated tubes has improved clotting behavior, making them suitable for tests requiring a glass-like surface. The integration of RFID technology into blood collection tubes allows for real-time tracking and seamless integration with laboratory information systems, minimizing sample mix-ups and enhancing traceability. AI?linked systems streamline data flow into laboratory information systems. Automation in tube assembly lines has also improved precision in additive insertion, sealing, and labeling.
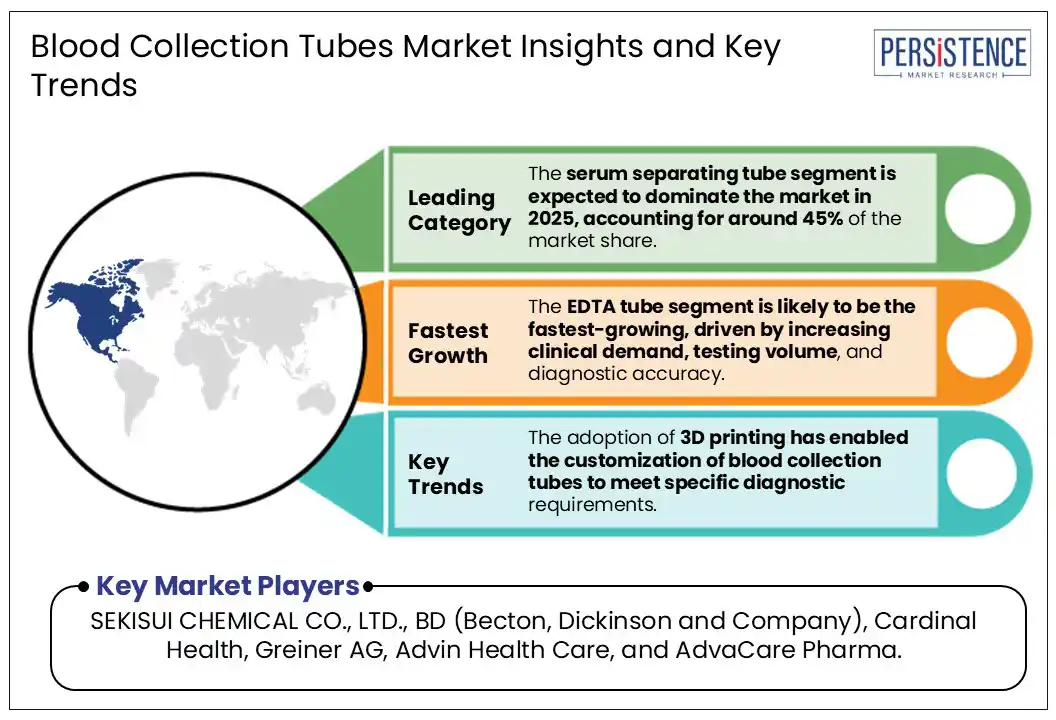
The adoption of 3D printing has enabled the customization of blood collection tubes to meet specific diagnostic requirements. This technology enables the creation of tubes with intricate internal structures, optimizing interactions between blood components and additives. Manufacturers are increasingly focused on eco-friendly materials such as biodegradable polymers and recycled plastics. Innovations such as nanotechnology and bioinformatics integration are anticipated to further create new opportunities.
By product, the serum separating tube segment is expected to dominate the market in 2025, accounting for around 45% of the market share. Made from transparent polypropylene or PMMA with color-coded silicone caps, SSTs contain a clot activator (micronized silica) and a separator gel with a density between serum and blood cells. These tubes are widely used in tests for blood glucose, liver & kidney function, electrolytes, hormones, and tumor markers. Their benefits include faster processing times, reduced contamination risks, and improved diagnostic precision. The rapid?serum tube segment (with thrombin activators) is the fastest-growing sub?segment for standard SSTs. Becton, Dickinson & Company (BD), Greiner Bio?One International GmbH, and Terumo Corporation are the major manufacturers.
The EDTA tube segment is likely to be the fastest-growing market in 2025, driven by increasing clinical demand, testing volume, and diagnostic accuracy. An EDTA tube is a blood collection vial containing ethylenediaminetetraacetic acid, an anticoagulant that prevents clotting. These tubes come in several color-coded stoppers, specifying different uses. EDTA have applications in hematology, immunohematology, toxicology, and molecular diagnostics. EDTA tubes are compatible with automated analyzers and are increasingly available in miniaturized formats (<2?mL) for pediatric and point-of-care use. Sarstedt AG, Greiner Bio?One, and Cangzhou Fukang Medical Supplies are the key manufacturers.
By end-user, the diagnostic center segment is expected to dominate the market with approximately 68% market share in 2025. This rapid growth is driven by the expanding popularity of preventive healthcare and routine check-ups, increased focus on early detection of chronic diseases and the proliferation of point-of-care testing services in outpatient settings. Diagnostic centers conduct a significant number of routine and specialized tests, including complete blood counts (CBCs), lipid profiles, and liver function tests, necessitating a consistent supply of blood collection tubes. The integration of automation and advanced diagnostic technologies in these centers enhances testing efficiency and accuracy. Labcorp, Quest Diagnostics, Metropolis Healthcare, Sonic Healthcare, and SYNLAB Group are key diagnostic centers.
The healthcare center segment is likely to grow the fastest in 2025. Healthcare centers encompass hospitals, clinics, and outpatient facilities. Healthcare centers perform a substantial number of diagnostic procedures, including routine blood tests and specialized assays, necessitating a consistent supply of blood collection tubes. Many hospitals operate in conjunction with blood banks, facilitating the collection, testing, and storage of blood products, thereby increasing the demand for blood collection tubes. The rise in complex surgeries and treatments, such as organ transplants and cancer therapies, has led to an increased need for blood collection for monitoring and transfusion purposes.
North America is estimated to dominate the market in 2025, accounting for a market share of 52% in 2025. An increase in chronic conditions such as diabetes, cardiovascular diseases, hematological conditions, and cancer is leading to a higher demand for blood tests, thereby driving the need for blood collection tubes. The aging population (especially in the U.S. and Canada) fuels higher utilization of lab services for managing geriatric disorders, necessitating frequent blood sample analysis. The FDA and Clinical Laboratory Improvement Amendments enforce strict quality controls for diagnostic devices, including blood collection tubes. Growth in preventive health checkups and home sample collection services (Everlywell, LetsGetChecked) also acts as a major driver.
The U.S. dominates the blood collection tubes market within North America due to the high incidence of bloodstream infections and chronic conditions, particularly among the elderly. According to CDC data, candidemia ranks as the fourth most common healthcare-associated bloodstream infection, affecting approximately 25,000 people annually. The country also sees robust blood donation activity, with over 6.8 million donors contributing about 13.6 million units each year. The growth of decentralized healthcare services and home-based diagnostics is increasing the demand for user-friendly and portable blood collection solutions.
Asia Pacific is likely to be the fastest-growing region in 2025. Chronic conditions such as diabetes, hypertension, cardiovascular diseases, and chronic kidney disease are increasing at an alarming rate, especially in countries including India, China, and Indonesia, driving the need for blood collection tubes for diagnostic purposes. The International Diabetes Federation reports that over 60% of the world’s diabetic population lives in Asia. Rapid growth in point-of-care testing and home diagnostics such as Thyrocare, 1mg, Redcliffe Labs, Sinocare Inc., and Arkray are driving growth. Government initiatives such as Make in India, China’s Health China 2030, and the Philippines’ Universal Health Care Act are promoting domestic production of blood collection tubes.
China's blood collection tubes market is experiencing significant growth. According to the latest Lancet study, China has the world’s largest diabetes population, accounting for nearly one-quarter of global cases. With one of the fastest aging populations globally, diabetes prevalence is expected to rise. Cancer rates are also increasing, prompting more regular blood testing for tumor markers, CBCs, and biochemical profiles. China’s “Healthy China 2030” plan aims to strengthen primary care, disease prevention, and early diagnosis. Expansion of independent clinical labs (KingMed and Adicon) and hospital modernization programs are also driving market growth. Wondfo Biotech, Improve Medical, and Jiangsu Kangjian Medical are scaling up production of EDTA tubes, serum separator tubes, and heparin tubes.
The blood collection tubes market in Europe is likely to experience significant growth in 2025. The International Diabetes Federation (IDF) estimates 66?million adults (20–79?years) in Europe to be living with diabetes. Europe is heavily investing in automated laboratory systems and digital diagnostics, increasing demand for barcoded, automation-compatible blood collection tubes. The implementation of the EU In Vitro Diagnostic Regulation (IVDR) in 2022 has significantly increased regulatory scrutiny for blood collection tubes. With one of the oldest populations globally, Europe sees high demand for routine blood testing to monitor comorbidities, organ function, and therapeutic levels.
In Europe, Germany is projected to exhibit the highest growth rate in the blood collection tubes market. Germany is experiencing a significant rise in chronic diseases, particularly type 2 diabetes, cardiovascular conditions, cancer, and neurodegenerative disorders. Under the EU Medical Device Regulation (MDR), these devices must bear the CE marking. Germany's Ordinance on Operators of Medical Devices (MPBetreibV) mandates that healthcare providers implement technological standards to ensure the safety and reliability of in vitro diagnostic devices.
The global blood collection tubes market is highly competitive with global leaders such as Becton, Dickinson & Company (BD), Greiner?Bio?One, Terumo Corporation, Qiagen, and Sarstedt, who leverage expansive product portfolios, advanced R&D (RFID-enabled smart tubes), and strong distribution networks across North America and Europe . Companies are investing in R&D and adopting growth strategies such as product innovations, strategic partnerships, and acquisitions.
The global blood collection tubes market is projected to be valued at US$ 5.2 Bn in 2025.
Recent innovations in blood collection tube technology have significantly enhanced diagnostic accuracy, patient safety, and laboratory efficiency.
The blood collection tubes market is poised to witness a CAGR of 7.2% from 2025 to 2032.
The adoption of 3D printing has enabled the customization of blood collection tubes to meet specific diagnostic requirements.
Major players in the global Blood Collection Tubes Market include SEKISUI CHEMICAL CO., LTD., BD (Becton, Dickinson and Company), Cardinal Health, Greiner AG, Advin Health Care, and AdvaCare Pharma.
|
Report Attribute |
Details |
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis |
Value: US$ Bn |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
|
Customization and Pricing |
Available upon request |
By Product
By Material
By End-user
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author