ID: PMRREP35695| 190 Pages | 8 Oct 2025 | Format: PDF, Excel, PPT* | Healthcare

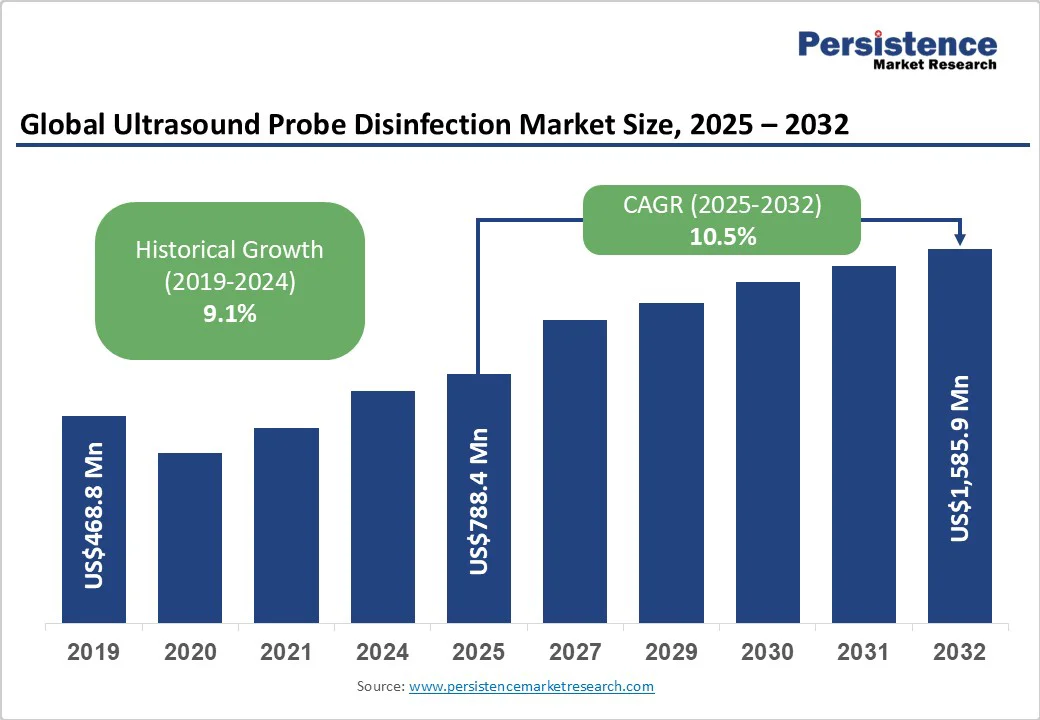
The global ultrasound probe disinfection market size is likely to be valued at US$788.4 Million in 2025 and is estimated to reach US$1,585.9 Million in 2032, growing at a CAGR of 10.5% during the forecast period 2025 - 2032, driven by the increasing incidence of hospital-acquired infections, highlighting the requirement for stringent disinfection protocols.
| Key Insights | Details |
|---|---|
| Ultrasound Probe Disinfection Market Size (2025E) | US$788.4 Mn |
| Market Value Forecast (2032F) | US$1,585.9 Mn |
| Projected Growth (CAGR 2025 to 2032) | 10.5% |
| Historical Market Growth (CAGR 2019 to 2024) | 9.1% |
The increasing prevalence of hospital-acquired infections (HAIs) is a key driver for improved ultrasound probe disinfection practices. The World Health Organization (WHO) notes that, on average, around 1 in 10 patients is affected by HAIs. Ultrasound probes, especially endocavitary ones, are classified as semi-critical devices and can become contaminated if not properly disinfected between uses.
For instance, studies have shown that despite the use of transducer covers, endocavitary transducers can remain contaminated with pathogens such as HPV unless subjected to high-level disinfection (HLD). This emphasizes the necessity for stringent disinfection protocols to mitigate the risk of HAIs. Healthcare facilities are hence adopting unique disinfection technologies such as automated systems and UV-C light to ensure thorough decontamination and improve patient safety.
The expanding use of ultrasound imaging in various medical specialties is pushing the demand for effective probe disinfection methods. Ultrasound is increasingly utilized for non-invasive diagnostic procedures and treatments across fields such as cardiology, obstetrics, and musculoskeletal medicine. As the frequency of ultrasound procedures rises, so does the potential for cross-contamination if disinfection protocols are inadequate.
It has led to the development and implementation of highly efficient disinfection technologies such as automated cleaning systems and chemical-free UV-C disinfection. These help to maintain hygiene standards and prevent the transmission of infections.
A key barrier to effective ultrasound probe disinfection is the variability in cleaning practices and insufficient staff training. Studies have exhibited that a substantial portion of healthcare professionals lack formal education on proper disinfection protocols. For instance, a recent survey revealed that 83% of respondents had no formal training in cleaning and maintaining ultrasound equipment, and 94% were unaware of established guidelines for probe cleaning.
The lack of standardized training leads to inconsistent practices such as improper cleaning techniques or failure to follow manufacturer instructions, which can compromise patient safety. The absence of uniform protocols increases the risk of healthcare-associated infections, emphasizing the requirement for comprehensive training programs and adherence to established guidelines.
Even when the main body of ultrasound probes is disinfected, the handles are often neglected, posing a significant infection risk. Research indicates that bacterial contamination is prevalent on probe handles, including pathogens responsible for hospital-acquired infections.
A study focusing on emergency departments found that ultrasound probes, specifically their handles, can harbor bacteria, potentially leading to nosocomial infections. This oversight highlights the necessity for comprehensive disinfection protocols that include all parts of the ultrasound equipment, ensuring thorough infection control and patient safety.
The adoption of UV-C technology for ultrasound probe disinfection is gaining momentum due to its chemical-free approach, which eliminates the risks associated with chemical exposure and residue. Devices such as Germitec's Chronos utilize UV-C light to achieve HLD in approximately 90 seconds, effectively targeting a broad spectrum of pathogens, including HPV and mycobacteria.
This method not only improves safety by reducing chemical handling but also refines workflows in clinical settings. The FDA's clearance of Chronos underscores the surging confidence in UV-C technology as a reliable and eco-friendly disinfection solution.
Automated systems for high-level disinfection (HLD) of ultrasound probes are transforming infection control practices in healthcare facilities. For instance, the Ethos Automated Ultrasound Probe Cleaner Disinfector integrates cleaning and HLD into a single device, approved for use with various ultrasound probes from manufacturers such as Philips, Samsung, and Siemens.
This integration reduces manual handling, minimizes human error, and ensures consistent disinfection outcomes. Such developments not only improve operational efficiency but also improve patient safety by adhering to stringent infection control standards.
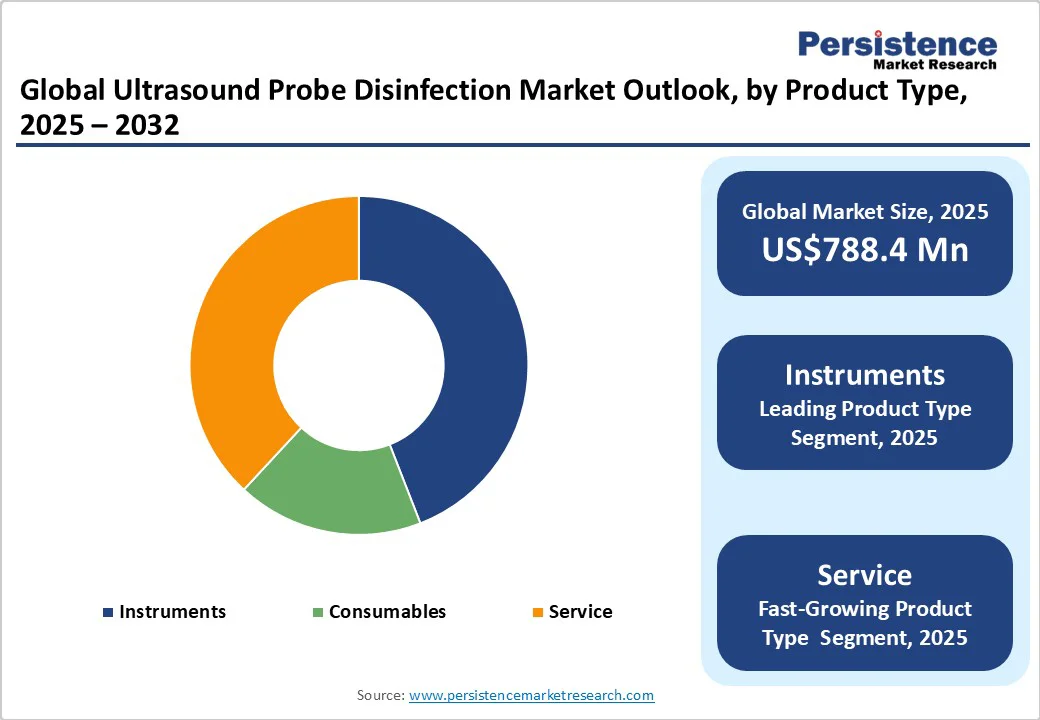
Instruments are anticipated to register a share of around 44.1% in 2025, due to their ability to provide consistent, HLD efficiently. Automated reprocessors, UV-C disinfectors, and manual soaking stations ensure thorough cleaning, which is important for preventing HAIs.
Services play a key role in ultrasound probe disinfection by providing specialized expertise and support. Companies such as Tristel deliver training, maintenance, and validation services to ensure compliance with infection control standards. These services help healthcare facilities maintain optimal disinfection practices, reduce operational risks, and extend the lifespan of ultrasound equipment.
The intermediate level disinfection/low-level disinfection segment is expected to hold nearly 63.2% of the market share in 2025, owing to its efficacy in eliminating most vegetative bacteria and viruses, including bloodborne pathogens such as HIV and hepatitis B and C. These methods are suitable for non-critical ultrasound probes that contact intact skin, such as those used in abdominal or musculoskeletal imaging. Their widespread use is attributed to their effectiveness, ease of application, and cost-efficiency.
HLD is essential for semi-critical ultrasound probes that come into contact with mucous membranes or non-intact skin, such as transvaginal or transrectal probes. HLD eliminates all microorganisms except for a few resistant bacterial spores, ensuring patient safety during procedures. The Centers for Disease Control and Prevention (CDC) and the American Institute of Ultrasound in Medicine (AIUM) recommend HLD for these devices to prevent the transmission of infections.
Hospitals and clinics are speculated to account for approximately 44.8% of the market share in 2025, backed by their high patient volumes and diverse range of procedures. These settings frequently utilize ultrasound for both diagnostic and therapeutic purposes, including high-risk procedures such as transvaginal and transrectal imaging. Such applications necessitate stringent infection control measures to prevent HAIs.
Diagnostic imaging centers play a key role in ultrasound probe disinfection due to their specialized focus on imaging procedures. These centers often perform a high volume of scheduled diagnostic exams, including obstetric and musculoskeletal imaging, where transducers are reused multiple times daily. The frequent use of ultrasound probes in these settings increases the risk of cross-contamination, making effective disinfection practices essential to ensure patient safety.
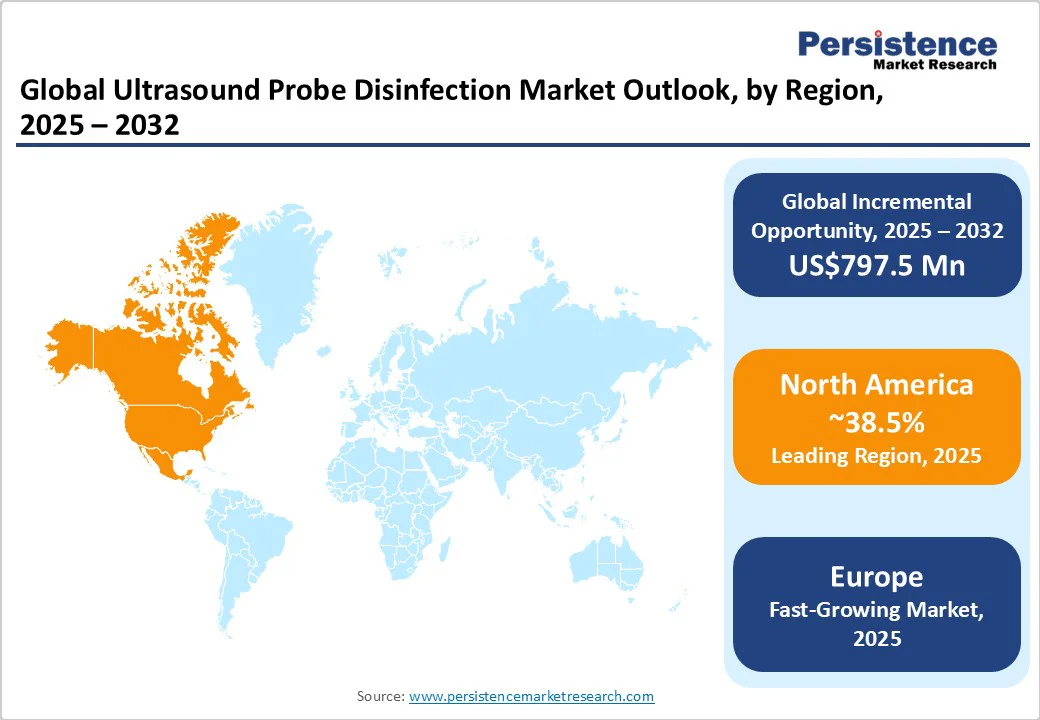
In 2025, North America is predicted to account for approximately 38.5% of the market share, as ultrasound probe disinfection is governed by stringent guidelines to mitigate the risk of HAIs. The American Institute of Ultrasound in Medicine (AIUM) categorizes ultrasound probes based on their clinical use, dictating the level of disinfection required. For instance, probes that contact mucous membranes or non-intact skin necessitate HLD, while those used on intact skin require low-level disinfection (LLD).
Healthcare facilities are extensively adopting unique disinfection technologies to improve patient safety and streamline workflows. For example, Germitec’s Chronos system utilizes UV-C technology to achieve HLD in just 90 seconds, delivering a chemical-free solution that reduces the risk of HAIs and supports environmental sustainability. However, studies indicate that over 80% of ultrasound probe handles remain contaminated when not properly disinfected, emphasizing the requirements for comprehensive cleaning protocols.
In Europe, ultrasound probe disinfection practices vary significantly across countries and institutions, leading to inconsistent infection control standards. A 2016 survey by the European Society of Radiology revealed that 29% of respondents did not disinfect ultrasound probes after standard surface examinations, 11% skipped disinfection after endo-cavity procedures, and 6% neglected to disinfect probes following interventional procedures.
Additionally, 11% failed to consistently use probe covers for endo-cavity exams, and 23% omitted them during interventional procedures. The survey also found that only 30% used sterile gel sachets during endo-cavity scans and 77.5% used sterile gel for interventional procedures. These findings highlight the requirement for standardized protocols and improved practitioner education to prevent cross-contamination and ensure patient safety.
In Asia Pacific, ultrasound probe disinfection practices are evolving, influenced by varying healthcare infrastructures and infection control standards across countries. While developed nations such as Japan and Australia have established comprehensive guidelines and strict disinfection protocols, other countries are working toward standardizing practices to improve patient safety.
The Asia Pacific Society of Infection Control (APSIC) has developed guidelines to assist healthcare facilities in achieving high standards in sterilization and disinfection. These guidelines aim to provide practical recommendations for the reprocessing of instruments in healthcare settings. This shows the importance of adhering to infection control measures to prevent cross-contamination and ensure patient safety.
The global ultrasound probe disinfection market is characterized by the presence of Nanosonics, Tristel Plc, STERIS, Ecolab, and Fortive Corporation. They deliver a wide range of disinfection solutions, including automated systems, consumables, and high-level disinfectants. Technological developments are a key trend in this market. For example, Nanosonics' Trophon2 system incorporates AcuTrace technology, facilitating digital documentation and workflow optimization for HLD.
Key business strategies in the ultrasound probe disinfection market focus on innovation, such as developing automated and digital-enabled systems, as well as expanding geographically in high-growth regions, including Asia Pacific. In addition, renowned companies are forming partnerships with hospitals and clinics, providing cost-effective consumables, emphasizing regulatory compliance, and embracing strong brand reputation to capture market share and improve customer loyalty.
The ultrasound probe disinfection market is projected to reach US$788.4 Million in 2025.
Rising hospital-acquired infections and increased use of ultrasound in diagnostics are the key market drivers.
The ultrasound probe disinfection market is poised to witness a CAGR of 10.5% from 2025 to 2032.
Expanding adoption in emerging healthcare sectors and the development of rapid disinfection solutions are the key market opportunities.
Nanosonics, ASP (Fortive), and Tristel Plc are a few key market players.
| Report Attribute | Details |
|---|---|
| Historical Data/Actuals | 2019 - 2024 |
| Forecast Period | 2025 - 2032 |
| Market Analysis | Value: US$ Mn |
| Geographical Coverage |
|
| Segmental Coverage |
|
| Competitive Analysis |
|
| Report Highlights |
|
By Product Type
By Method
By End-user
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author