ID: PMRREP33839| 210 Pages | 16 Jan 2026 | Format: PDF, Excel, PPT* | Healthcare

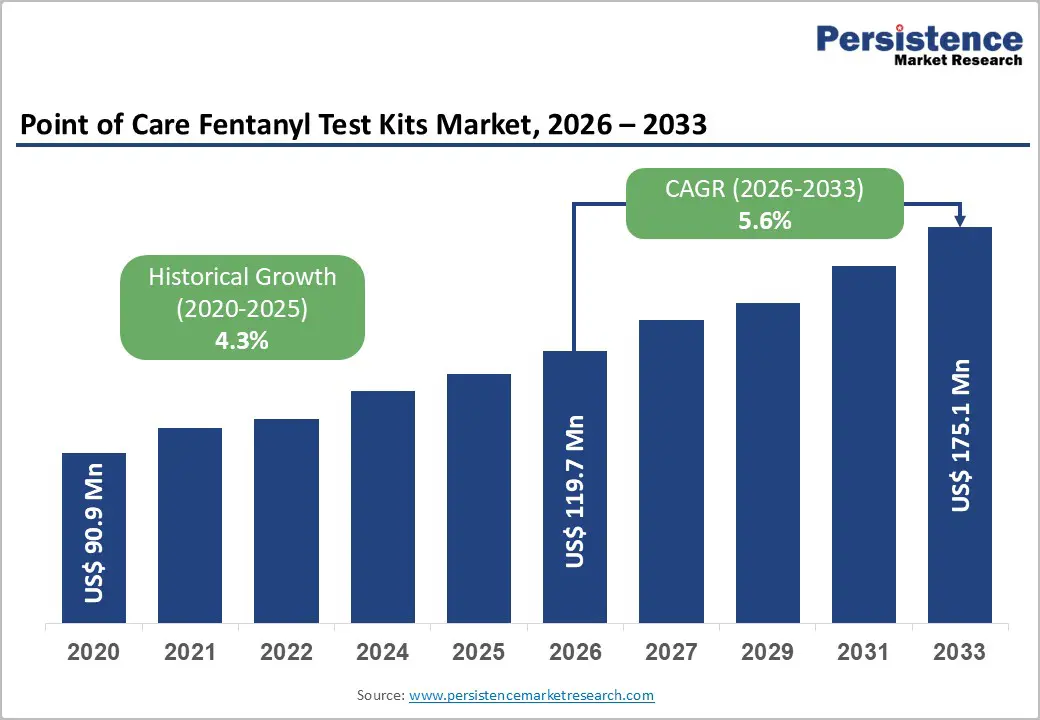
The global point-of-care fentanyl test kits market size is estimated to grow from US$119.7 million in 2026 to US$ 175.1 million by 2033, growing at a CAGR of 5.6% during the forecast period from 2026 to 2033.
The market is witnessing strong growth due to rising fentanyl abuse, increasing opioid overdose deaths, and growing demand for rapid on-site drug detection. These kits enable quick, reliable screening of fentanyl in urine, saliva, or drug residues across healthcare, forensic, and harm-reduction settings. Widespread adoption in emergency departments, diagnostic laboratories, law enforcement agencies, and community outreach programs is supporting market expansion. Technological advancements improving sensitivity and ease of use further enhance uptake.
| Key Insights | Details |
|---|---|
| Point of Care Fentanyl Test Kits Market Size (2026E) | US$119.7 Mn |
| Market Value Forecast (2033F) | US$175.1 Mn |
| Projected Growth (CAGR 2026 to 2033) | 5.6% |
| Historical Market Growth (CAGR 2020 to 2025) | 4.3% |
The rise of fentanyl analog contamination in non-opioid drugs has emerged as a critical and unique driver for the point-of-care fentanyl test kits Market. Traditionally associated with heroin and prescription opioids, fentanyl is now increasingly detected in substances such as cocaine, methamphetamine, MDMA, and counterfeit prescription pills. This shift has significantly expanded the at-risk population, as many users of these substances do not expect opioid exposure and therefore lack tolerance, substantially increasing the risk of overdose and fatal outcomes. The unpredictable nature of illicit drug supply chains, combined with the proliferation of potent fentanyl analogs, has intensified the need for rapid detection at the point of use or care.
Point-of-care fentanyl test kits address this emerging challenge by enabling immediate, on-site screening of drugs or biological samples without reliance on laboratory infrastructure. Their portability and ease of use allow deployment across emergency departments, law enforcement field operations, harm-reduction programs, and community outreach initiatives. As awareness grows around fentanyl contamination beyond traditional opioid markets, healthcare providers and public health agencies are prioritizing early detection to guide timely intervention and overdose prevention strategies. Additionally, the spread of fentanyl into counterfeit pills resembling commonly prescribed medications has heightened demand for rapid testing among younger and recreational users. This expanding scope of fentanyl exposure across diverse drug categories is directly accelerating the adoption of point-of-care test kits, positioning them as essential tools in modern drug safety and overdose prevention efforts.
One of the most critical restraints in the Point-of-Care fentanyl test kits market is the risk of false-negative results caused by extremely low fentanyl concentrations in tested samples. Fentanyl is a highly potent synthetic opioid, often present in trace amounts that may fall below the detection threshold of many rapid test kits. Even minute quantities can cause severe respiratory depression or fatal overdose, making undetected exposure particularly dangerous. When test kits fail to identify such low concentrations, users may incorrectly assume a substance is safe, significantly increasing overdose risk.
Inconsistent drug mixing practices in illicit drug manufacturing and distribution further amplify this limitation. Fentanyl is often unevenly distributed within a substance, meaning a small test sample may not accurately represent the overall composition. As a result, individuals relying on point-of-care kits may receive negative results despite the presence of fentanyl elsewhere in the drug. In clinical settings, false negatives can delay appropriate medical intervention, impacting patient outcomes during suspected overdose or poisoning cases.
Additionally, sample dilution methods required for testing can further reduce detectable fentanyl levels, especially when users lack proper guidance or training. Environmental factors such as improper storage, expired kits, or exposure to heat and humidity may also reduce test sensitivity over time. These challenges collectively undermine confidence in point-of-care testing, underscoring the need for improved sensitivity, standardized sampling protocols, and complementary confirmatory testing to mitigate the risks of false-negative results.
The development of multiplex and multi-drug panels represents a significant opportunity within the point-of-care (POC) fentanyl test kits market. Unlike traditional single-analyte kits that detect only fentanyl, multiplex panels are designed to simultaneously screen for multiple opioids and controlled substances, including heroin, morphine, oxycodone, and emerging synthetic analogs. This innovation enhances the utility of POC testing by providing comprehensive results from a single sample, whether urine, saliva, or drug residue, reducing the need for multiple separate tests and saving critical time in both clinical and emergency scenarios.
Such panels are particularly valuable in emergency departments, forensic laboratories, rehabilitation centers, and harm-reduction programs, where rapid identification of polydrug exposure is essential for informed decision-making. For instance, clinicians can tailor treatment strategies in real time, law enforcement can more accurately assess illicit drug composition in street seizures, and outreach programs can provide better guidance to at-risk populations.
Technological advancements, such as lateral flow immunoassays, microfluidic platforms, and high-sensitivity antibody reagents, are enabling these multiplex tests to achieve high specificity and accuracy despite the complex nature of mixed drug samples. Additionally, these panels can be integrated with digital reporting systems, allowing automated data capture, trend analysis, and improved regulatory compliance.
Overall, multiplex and multi-drug POC panels not only streamline testing workflows but also enhance public health outcomes, reduce accidental overdoses, and support broader opioid monitoring initiatives, making them a transformative innovation in the fentanyl detection market.
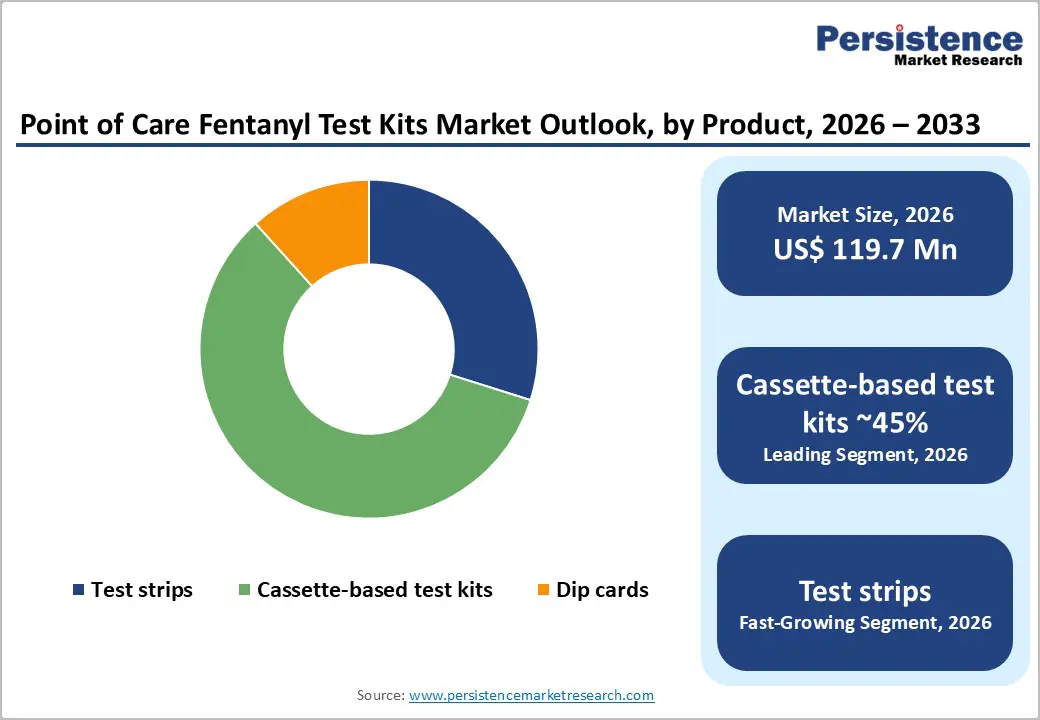
Among the various formats of point-of-care fentanyl test kits, cassette-based test kits dominate the market due to their combination of accuracy, reliability, and versatility, which meet the demands of both clinical and emergency applications. Unlike simple test strips or dip cards, cassette-based kits integrate lateral flow immunoassay technology within a compact device, allowing for controlled sample flow and consistent reaction conditions, which significantly reduces the risk of user error. This design ensures high sensitivity and specificity, even when detecting low concentrations of fentanyl or its analogs, making it suitable for critical healthcare settings such as hospitals, emergency departments, and diagnostic laboratories where accurate results are paramount.
Additionally, cassette-based kits are highly portable and easy to handle, yet robust enough for forensic or field use, bridging the gap between laboratory-grade testing and rapid on-site screening. Their format also supports integration with digital readers or reporting systems, enabling automated documentation and real-time data capture, which is increasingly valued in regulatory-compliant workflows and public health monitoring.
In contrast, test strips and dip cards, while inexpensive and convenient for harm-reduction programs or personal use, often lack the precision, durability, and standardized reporting features required in professional environments. This combination of technical superiority, operational versatility, and compatibility with institutional protocols firmly establishes cassette-based kits as the market leader in point-of-care fentanyl detection.
Hospitals and emergency departments (EDs) represent the largest end-user segment in the point-of-care (POC) fentanyl test kits market, given the critical nature of patient care in these settings. Fentanyl, being a highly potent synthetic opioid, can cause rapid-onset respiratory depression and overdose, making immediate detection essential. Emergency departments are often the first point of contact for patients presenting with suspected opioid exposure or overdose. The availability of POC test kits enables healthcare professionals to quickly identify fentanyl presence, allowing timely administration of antidotes, supportive care, or critical interventions, which can significantly reduce mortality rates.
Moreover, hospitals maintain high patient volumes, requiring frequent and routine testing of at-risk individuals, which drives sustained demand for reliable test kits. The cassette-based and rapid test formats preferred in clinical settings offer high accuracy, reproducibility, and ease of integration into existing workflows, aligning with hospitals’ operational requirements and regulatory standards.
In contrast, diagnostic and forensic laboratories primarily handle confirmatory or specialized testing and do not require the same rapid turnaround, resulting in lower volume consumption. Other end-users such as rehabilitation centers, law enforcement agencies, and community outreach programs, while important for preventive and harm-reduction efforts, face budget constraints and lower testing frequency, limiting their market share.
Overall, the combination of urgent clinical need, high testing frequency, regulatory compliance, and operational integration positions hospitals and emergency departments as the dominant segment in the POC fentanyl test kits market.
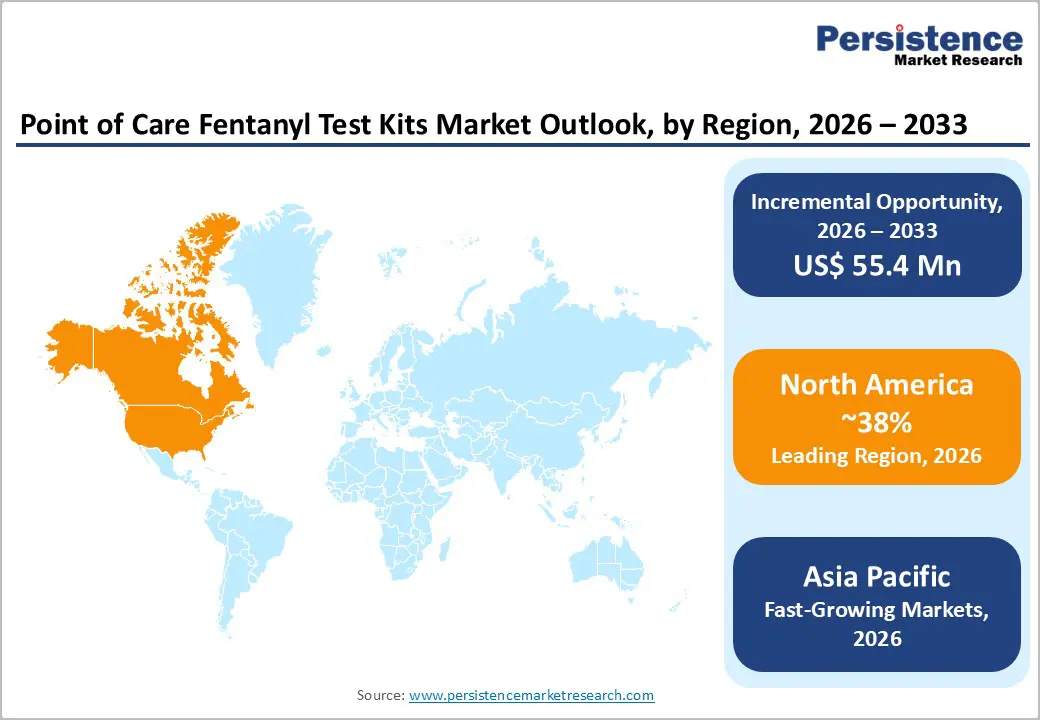
North America leads the global point-of-care (POC) fentanyl test kits market, driven primarily by the opioid epidemic in the United States, where synthetic opioids like fentanyl account for a significant proportion of overdose deaths. Hospitals, emergency departments, and forensic laboratories in the U.S. increasingly rely on rapid test kits for immediate identification of fentanyl exposure, enabling timely clinical interventions and reducing mortality rates. Widespread awareness campaigns, stringent regulatory frameworks, and government funding for overdose prevention programs have further accelerated the adoption of POC testing solutions.
Technological innovations, such as high-sensitivity lateral flow assays, cassette-based kits, and multiplex panels, are widely implemented across North American healthcare systems to enhance detection accuracy and operational efficiency. Additionally, harm-reduction organizations and community outreach programs in the U.S. distribute test strips for street drugs, reflecting a growing trend toward preventive testing outside clinical environments.
The U.S. market also benefits from established healthcare infrastructure, advanced laboratory networks, and robust reimbursement policies, which facilitate large-scale procurement and integration of POC fentanyl test kits. With ongoing government initiatives targeting opioid overdose mitigation and increased adoption in emergency medical services, North America is expected to maintain its dominant market position while influencing technological and regulatory trends globally.
The Asia Pacific region is emerging as a high-growth market for point-of-care (POC) fentanyl test kits, driven by rising awareness of opioid abuse, expanding healthcare infrastructure, and increasing adoption of rapid diagnostic technologies. While historically the prevalence of synthetic opioid misuse was lower than in North America, recent reports indicate a growing incidence of fentanyl-related substance abuse and accidental overdoses in countries like China, India, Japan, and Australia, prompting governments and healthcare institutions to prioritize early detection and prevention strategies.
Hospitals, emergency departments, and diagnostic laboratories are increasingly adopting POC test kits to facilitate rapid, on-site detection of fentanyl, reducing dependence on centralized laboratory testing and improving clinical response times. Multiplex and cassette-based kits are gaining traction due to their accuracy, portability, and ease of integration into hospital workflows, while dip cards and test strips are being introduced in community health initiatives and harm-reduction programs targeting at-risk populations.
Supportive government policies, growing public health funding, and initiatives by non-governmental organizations are enhancing market accessibility and affordability. Additionally, technological collaborations with global manufacturers are enabling local production and distribution, thereby reducing costs and improving supply chain efficiency. These factors collectively position Asia Pacific as a rapidly expanding market, offering significant opportunities for manufacturers to introduce innovative POC fentanyl testing solutions and address unmet clinical and community needs across the region.
The competitive landscape of the point-of-care fentanyl test kits market is characterized by intense competition driven by innovation, product differentiation, and technological advancements. Key players focus on developing high-sensitivity, rapid, and user-friendly test formats, including cassette-based kits, test strips, and multiplex panels, to enhance market share. Strategic initiatives such as partnerships, collaborations, and regional expansions are common to strengthen distribution networks and reach diverse end-users across hospitals, emergency departments, forensic labs, and community programs.
The global point of care fentanyl test kits market is projected to be valued at US$119.7 Mn in 2026.
Increasing incidents of fentanyl-related overdoses and deaths globally have created an urgent need for rapid, on-site detection solutions.
The global market is poised to witness a CAGR of 5.6% between 2026 and 2033.
Growing acceptance of self-testing and harm-reduction initiatives opens opportunities for user-friendly, non-clinical test kits.
BTNX Inc., The Bunk Police, DanceSafe, Instanosis Inc., Ovus Medical, and others.
| Report Attribute | Details |
|---|---|
| Historical Data/Actuals | 2020 - 2025 |
| Forecast Period | 2026 - 2033 |
| Market Analysis | Value: US$ Mn |
| Geographical Coverage |
|
| Segmental Coverage |
|
| Competitive Analysis |
|
| Report Highlights |
|
By Product Type
By Sample Type
By End-user
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author