ID: PMRREP32736| 191 Pages | 2 Dec 2025 | Format: PDF, Excel, PPT* | Healthcare

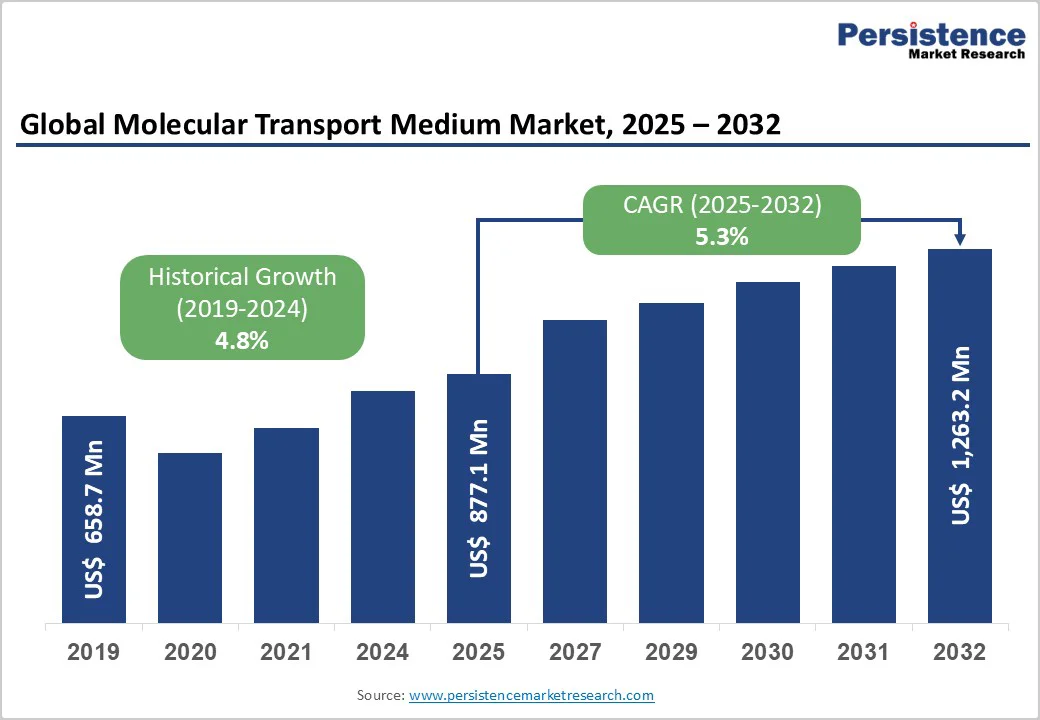
The global molecular transport medium market size is valued at US$877.1 million in 2025 and is projected to reach US$1,263.2 million, growing at a CAGR of 5.3% during the forecast period from 2025 to 2032. The global molecular transport medium (MTM) market is growing steadily as demand for accurate and contamination-free sample collection increases across diagnostic laboratories, hospitals, and research institutions.
MTMs support the safe stabilization and transport of viral, bacterial, and molecular samples, driven by rising infectious disease surveillance, the expansion of molecular diagnostics, and the growth of decentralized testing. Technological advancements in nucleic acid preservation, improved cold-chain logistics, and wider adoption in PCR and sequencing workflows further boost market uptake.
| Key Insights | Details |
|---|---|
| Molecular Transport Medium Market Size (2025E) | US$877.1 Mn |
| Market Value Forecast (2032F) | US$1,263.2 Mn |
| Projected Growth (CAGR 2025 to 2032) | 5.3% |
| Historical Market Growth (CAGR 2019 to 2024) | 4.8% |
Technological improvements have resulted in a typical shift away from traditional transport mediums and toward innovative and effective molecular transport mediums that are safer and convenient. New products developed are expected to aid in the decreased risk of exposure using a non-hazardous and leak-proof formulation during transport, storage, and processing.
In June 2020, bioBoaVista introduced a viral transport medium (MTV) for more effective COVID-19 nasal examinations. Its unique formulation prevents other pathogens from interfering with the virus's stability through competition and contamination.
Furthermore, the growing need to preserve sample stability during transport and storage is another crucial factor. After collecting the sample, it is necessary to store it promptly to prevent the degradation or death of viable cells.
Specimen samples are mostly frozen at -20°C or lower and brought to the testing laboratory in ice packs if held for extended durations. Repeated freezing and thawing of specimen samples for testing may compromise their stability. Thus, molecular transport media are used to help keep specimen samples stable during storage and transportation for approximately 48 hours.
Various media have been recommended for stabilizing specimens to detect viruses, bacteria, and fungi, primarily during diagnostic examinations. This media is usually created using saline solutions or balanced salt solutions with a buffering capacity to maintain a neutral pH.
To improve the consistency of viruses, researchers recommend a range of Plant-based protein supplements. Although some laboratories prepare in-house viral transport media, commercial formulations are widely used and supplied with sample collection kits containing sterile swabs.
The severity of seasonal infections such as flu and pandemics determines the demand for molecular transport mediums. Furthermore, numerous public awareness programs are carried out around the world, which have helped amplify public knowledge and, as a result, increased testing rates.
Seasonal influenza and other diseases are unexpected, and WHO estimates are sometimes inflated, resulting in a demand-supply gap. Furthermore, due to the unpredictable nature of infections, several kit manufacturing companies have adopted a conservative strategy, resulting in decreased supply even during pandemics or follow-up eruptions.
Home-testing-compatible MTMs present a significant market opportunity as consumers increasingly adopt self-collection kits for viral, bacterial, and genetic testing. These media must ensure high nucleic acid stability, pathogen inactivation, and simple handling without the need for professional supervision. Manufacturers can innovate with leak-proof packaging, pre-measured reagent volumes, and ambient-temperature stability to improve usability.
Integration with telehealth platforms and digital reporting systems further enhances convenience and accuracy. As home-based molecular testing expands across respiratory diseases, STI screening, pharmacogenomics, and wellness genomics, demand for reliable, consumer-safe MTMs will surge, enabling broader access to diagnostics and reducing pressure on centralized laboratories.
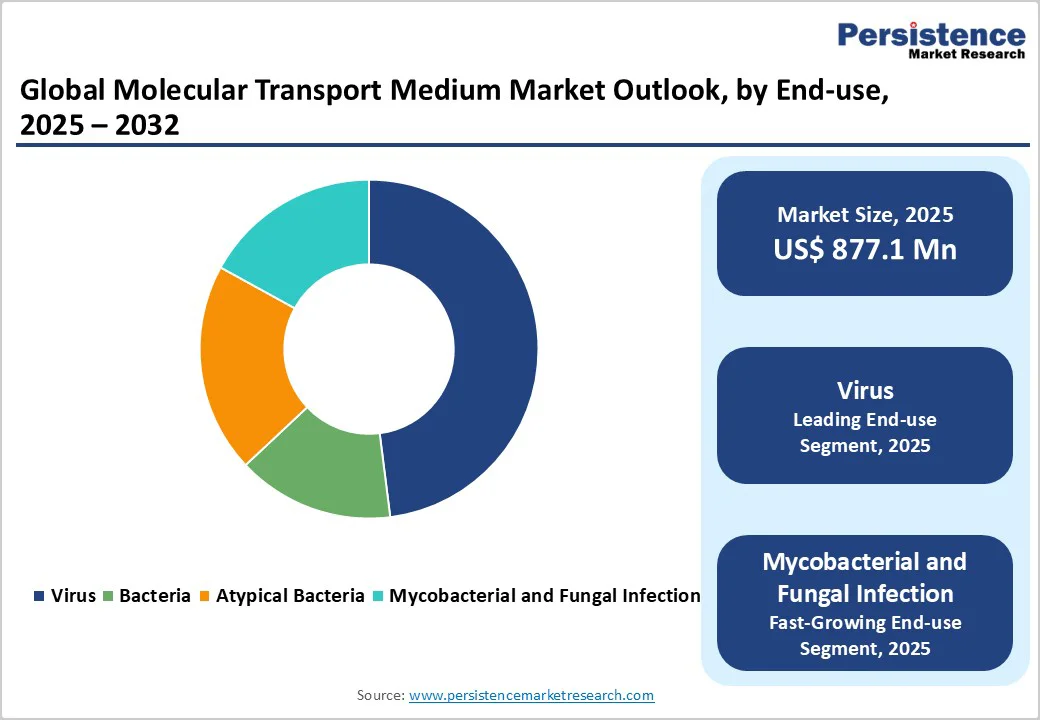
Inactivated Transport Medium holds the highest market share because it immediately neutralizes pathogens, making sample handling and transportation significantly safer for clinicians, lab technicians, and logistics personnel.
By reducing biosafety risks, it minimizes the need for high-level containment measures and simplifies compliance with transport regulations. Inactivated MTMs also preserve nucleic acids effectively, supporting accurate PCR and sequencing results, and often enable ambient-temperature stability, reducing cold-chain dependence.
Their broad adoption in respiratory virus testing, outbreak surveillance, and large-scale screening programs further drives demand, making them the preferred choice for healthcare systems and diagnostic laboratories worldwide.
By End-user Insights
Diagnostic laboratories account for the largest share of the Molecular Transport Medium (MTM) market because they handle the highest volume of molecular tests across viral, bacterial, and genetic applications. These labs require highly reliable transport media to maintain nucleic acid integrity, prevent contamination, and support high-throughput PCR and sequencing workflows.
With strict quality standards, validated protocols, and continuous sample inflow from hospitals, clinics, and public health programs, diagnostic labs rely heavily on consistent MTM supply. Their central role in disease surveillance, outbreak management, and routine molecular testing makes them the primary consumers, driving their dominant market share.
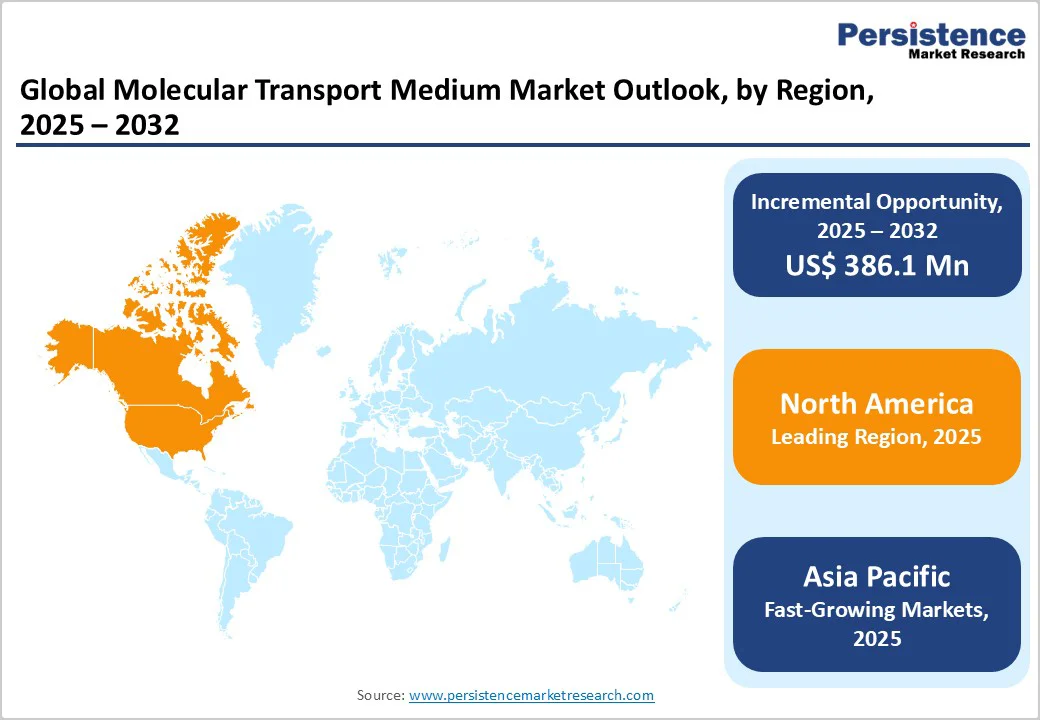
North America leads the molecular transport medium (MTM) market due to its advanced diagnostic infrastructure, high adoption of molecular testing, and strong investment in infectious disease surveillance. The U.S. conducts one of the world’s largest volumes of PCR and sequencing tests annually, supported by extensive laboratory networks and public health programs.
The growing use of MTMs in respiratory virus screening, antimicrobial resistance monitoring, and decentralized/home-based collection kits is strengthening regional demand. Continuous FDA oversight encourages quality-certified MTMs, while large players and biotech innovators accelerate product development. Expanding genomic surveillance initiatives and strong preparedness funding further reinforce North America’s dominant market position.
Asia Pacific molecular transport medium (MTM) market is growing rapidly due to expanding molecular diagnostics capacity, rising infectious disease burdens, and increased government investment in public health infrastructure. Countries like China, India, and Japan are scaling PCR, sequencing, and genomic surveillance programs, driving higher MTM consumption.
Growing awareness of early pathogen detection, improved laboratory networks, and the adoption of decentralized testing models further support demand. Local manufacturers are expanding production to reduce import dependence, while innovation in ambient-temperature MTMs addresses logistical challenges in rural regions. Rising focus on respiratory viruses, AMR monitoring, and clinical research continues to accelerate regional market growth.
The Molecular Transport Medium (MTM) market is moderately competitive, with global diagnostics companies, specialized media manufacturers, and regional laboratory suppliers actively expanding their portfolios.
Leading players focus on nucleic acid stabilization technologies, pathogen inactivation, and automation-friendly formulations to differentiate their offerings. Strategic partnerships with diagnostic kit manufacturers, hospitals, and public health agencies strengthen market reach.
The global molecular transport medium market is valued at US$877.1 Mn in 2025.
Growth of self-collection kits and remote diagnostics requires safe, stable MTMs.
The global market is poised to witness a CAGR of 5.3% between 2025 and 2032.
Specialized media supporting genomic surveillance and metagenomic sequencing workflows.
Thermo Fisher Scientific Inc., Becton, Dickinson, and Company, Cardinal Health, Quidel Corporation, and others.
| Report Attribute | Details |
|---|---|
| Historical Data/Actuals | 2019 - 2024 |
| Forecast Period | 2025 - 2032 |
| Market Analysis | Value: US$ Mn |
| Geographical Coverage |
|
| Segmental Coverage |
|
| Competitive Analysis |
|
| Report Highlights |
|
By Product Type
By Application
By Sample
By End-user
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author