ID: PMRREP34560| 243 Pages | 2 Feb 2026 | Format: PDF, Excel, PPT* | Healthcare

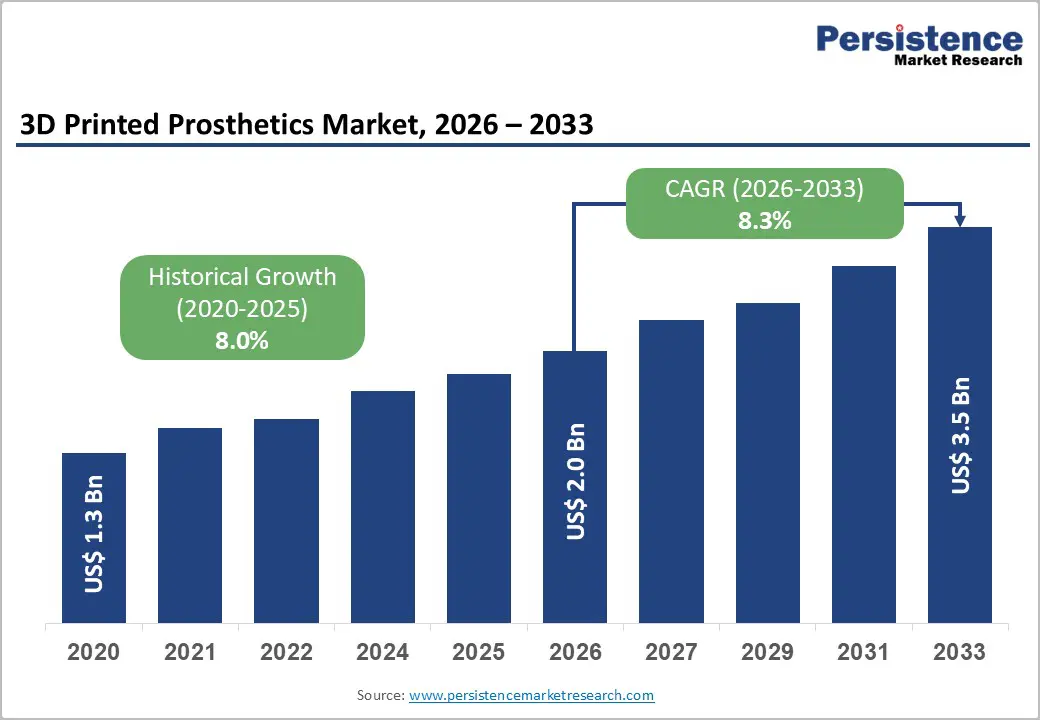
The global 3D printed prosthetics market size is likely to be valued at US$2.0 billion in 2026 and is expected to reach US$3.5 billion by 2033, growing at a CAGR of 8.3% during the forecast period from 2026 to 2033, driven by rapid advancements in additive manufacturing technologies, which allow for the production of highly customized, lightweight, and cost-effective prosthetic devices that are tailored to individual anatomical and functional needs.
Rising cases of limb loss from trauma, diabetes, vascular disorders, and congenital conditions are boosting global demand for prosthetics. Advances in biocompatible materials, 3D scanning, and CAD software are improving durability, comfort, and mobility. Localized production and point-of-care 3D printing are increasing accessibility, cutting lead times, and reducing material waste.
| Key Insights | Details |
|---|---|
| 3D Printed Prosthetics Market Size (2026E) | US$2.0 Bn |
| Market Value Forecast (2033F) | US$3.5 Bn |
| Projected Growth (CAGR 2026 to 2033) | 8.3% |
| Historical Market Growth (CAGR 2020 to 2025) | 8.0% |
Traditional prosthetic manufacturing involves labor-intensive processes and standardized designs that often fail to meet individual anatomical requirements. With 3D printing, manufacturers can now create prosthetic components tailored precisely to the patient’s residual limb, ensuring better fit, comfort, and mobility. Technologies such as stereo lithography (SLA), selective laser sintering (SLS), and fused deposition modeling (FDM) allow for rapid prototyping, iterative design improvements, and the use of multi-material prints, combining strength, flexibility, and biocompatibility in a single device. This level of customization also extends to aesthetic features, allowing patients to select colors, textures, and designs that reflect personal preferences, thereby improving psychological acceptance and adoption.
Customization powered by 3D printing is a critical growth driver as it addresses the diverse functional and medical needs of prosthetic users. Each patient’s limb anatomy, residual muscle strength, and activity level differ, necessitating bespoke solutions that conventional prosthetics cannot easily provide. 3D printing allows for precise tailoring of socket shapes, limb lengths, and joint components, optimizing comfort, load distribution, and joint mobility. The ability to rapidly prototype and modify designs based on patient feedback accelerates rehabilitation and enhances user satisfaction. 3D printing facilitates the integration of lightweight, durable polymers such as polypropylene, polyethylene, and advanced biocompatible materials, improving the overall performance and lifespan of prosthetic devices. This technology also enables decentralized production models, where clinics or rehabilitation centers can print devices on-site, reducing delivery time and costs while improving access in remote or underserved regions.
Prosthetics are classified as medical devices, requiring compliance with strict regulatory standards such as the FDA in the U.S., CE marking in Europe, and ISO certifications internationally. These frameworks ensure patient safety and product reliability but can prolong approval timelines, particularly for novel 3D-printed designs that employ advanced materials or incorporate multi-material components. Regulatory bodies often require extensive clinical testing, biocompatibility assessments, and documentation of manufacturing processes, which can increase development costs and limit smaller manufacturers’ ability to enter the market. Lack of standardized guidelines for additive manufacturing in prosthetics creates uncertainty, as approval processes may vary by region.
Quality assurance challenges restrain market growth by introducing risks related to device reliability, patient safety, and long-term performance. 3D printed prosthetics rely on precise material properties, dimensional accuracy, and structural integrity, all of which must be rigorously tested to ensure consistent functionality. Variability in printers, materials, and post-processing techniques can lead to defects or inconsistencies, potentially compromising fit, durability, and comfort. Unlike traditional manufacturing, additive manufacturing often requires new testing protocols and validation procedures, increasing operational complexity for producers. Decentralized production models, such as on-site printing in clinics, introduce additional quality control concerns, as smaller facilities may lack standardized equipment calibration and skilled personnel.
The unique needs of children require customized and adaptable devices. Traditional prosthetic solutions often struggle to accommodate the rapid growth of pediatric patients, necessitating frequent replacements and adjustments, which increases costs and reduces accessibility. 3D printing addresses these challenges by enabling rapid, cost-effective production of lightweight, durable prosthetics that can be tailored to the child’s anatomy and functional requirements. Customization extends beyond fit to include aesthetics and design preferences, allowing children to feel confident and engaged with their prosthetic devices. Advanced 3D printing technologies facilitate iterative modifications, enabling clinicians to adjust components as the child grows without extensive delays or expense.
Personalized prosthetic solutions offer a broader market opportunity beyond pediatric care, targeting adult users with unique anatomical, functional, or lifestyle requirements. 3D printing enables highly individualized devices, from sockets and limb components to joints, designed to match a patient’s residual limb geometry, activity level, and specific rehabilitation goals. Such customization improves comfort, reduces pressure sores, and enhances long-term device usability, driving higher adoption rates among patients who previously struggled with off-the-shelf solutions. Personalization extends to materials selection, allowing the integration of lightweight polymers, flexible composites, or multi-material components that balance durability with mobility.
Sockets are expected to lead the 3D printed prosthetics market, accounting for approximately 46% of revenue in 2026, driven by their critical role in ensuring a precise fit, comfort, and stability for prosthetic users. The socket interfaces directly with the residual limb, distributing weight and pressure effectively, which is essential for mobility and long-term usability. 3D printing technology enhances socket design by enabling customized anatomical shapes and personalized adjustments based on patient-specific scans. For example, UNYQ, a leading prosthetic manufacturer, leverages additive manufacturing to produce tailored sockets that improve comfort while allowing aesthetic customization for individual users. These innovations reduce fitting errors and minimize the need for repeated adjustments, making sockets the most significant contributor to market revenue.
Limbs are likely to represent the fastest-growing segment in 2026, supported by the increasing demand for both upper and lower extremity replacements due to rising incidences of amputations from diabetes, trauma, and vascular diseases worldwide. 3D printing enables rapid prototyping and cost-effective production of limb components, making these solutions more accessible to patients in both developed and emerging markets. For example, Open Bionics has gained recognition for producing affordable, customizable bionic arms using 3D printing technology, offering patients enhanced mobility and functionality at a fraction of traditional costs. The ability to integrate lightweight materials such as polypropylene or advanced polymers allows for durable yet comfortable limb designs that can be quickly modified based on user feedback.
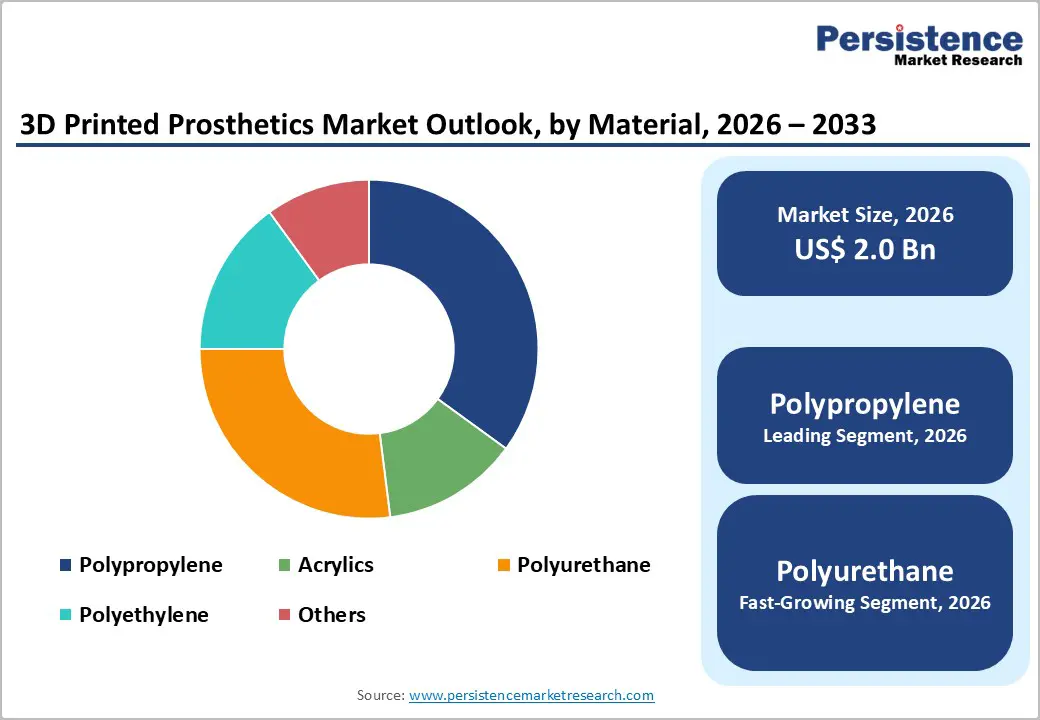
Polypropylene is projected to lead the market, capturing around 39% of the revenue share in 2026, supported by its lightweight, durable, and fatigue-resistant properties, which are crucial for the comfort and longevity of prosthetic devices. Polypropylene is also compatible with sterilization processes, ensuring patient safety and hygiene, which is particularly important in clinical settings. For example, 3D Systems Corporation utilizes polypropylene in the production of both sockets and limb components, providing cost-effective and functional solutions that meet the demands of hospitals and rehabilitation centers. The material’s ease of printing with additive manufacturing technologies allows for precise customization of prosthetic geometries, including complex lattice structures and ergonomically designed surfaces.
Polyurethane is likely to be the fastest-growing material, driven by its exceptional flexibility, durability, and patient comfort. TPU combines the elastic properties of rubber with the mechanical strength of plastics, making it especially suitable for components that require both softness for comfort and resilience for everyday use. Flexible TPU liners and inner socket layers improve the interface between the residual limb and the prosthetic device, reducing pressure points and enhancing long?term wearability. For example, Forward AM’s Ultrasint® TPU has been used to produce 3D printed prosthetic sockets that offer a customized, lightweight, yet durable solution, providing patients with better comfort and adaptability compared to traditional rigid materials.
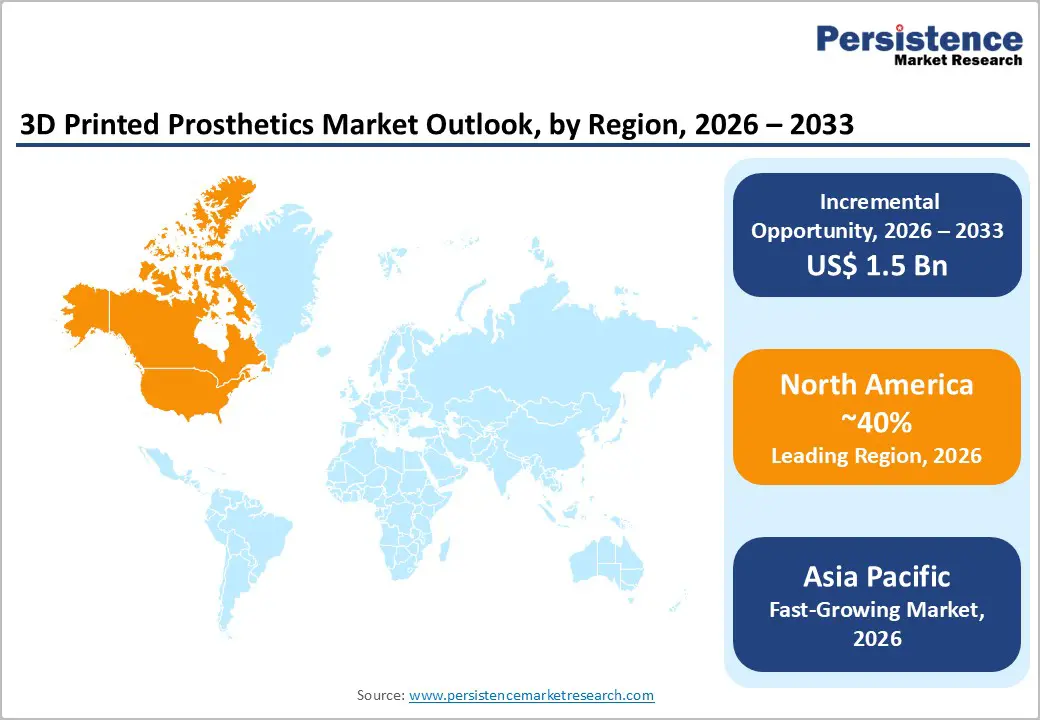
North America is anticipated to be the leading region, accounting for a market share of 40% in 2026, driven by advanced healthcare infrastructure, strong R&D ecosystems, and increasing clinical adoption of additive manufacturing for personalized medical devices. The United States, in particular, leads market growth due to a high incidence of amputations from diabetes, trauma, and vascular diseases, which fuels demand for lightweight, affordable, and patient-specific devices. Healthcare providers increasingly deploy point-of-care 3D printing workflows that integrate digital scanning, design, and production to reduce turnaround times and improve clinical outcomes for patients needing rapid prosthetic deployment.
Specific companies are shaping the North American competitive landscape through technology innovations and tailored solutions. For example, Limbitless Solutions, a U.S.based nonprofit organization, has developed open-source, 3D-printed bionic arms for children, significantly lowering costs compared with traditional prosthetics and making personalized devices accessible to a wider population. Limbitless’s model demonstrates how community-led innovation and additive manufacturing can drive social impact while advancing prosthetic capabilities in clinical and educational settings. Growing interest in multi-material prosthetics, improved biocompatible materials, and enhanced design software is also encouraging new product introductions that address both functional performance and patient comfort.
Europe is likely to be a significant market for 3D printed prosthetics in 2026, due to robust healthcare infrastructure, well-established rehabilitation services, and supportive medical device regulations. The region accounts for a significant portion of the market, reflecting strong adoption across countries such as Germany, the U.K., and France. European healthcare systems are increasingly integrating additive manufacturing for personalized prosthetic solutions that enhance patient function and comfort. Regulatory frameworks such as the EU medical device regulation (MDR) provide clear standards for safety, traceability, and technical documentation, encouraging manufacturers to innovate while ensuring compliance.
European companies and technology developers are supporting this market evolution by embedding additive manufacturing capabilities into orthopedic and prosthetic workflows. For example, Ottobock SE & Co. KGaA, a German orthopaedic technology leader with a reputation in prosthetics, has embraced digital manufacturing and research into advanced prosthetic components. Ottobock’s integration of 3D-assisted design and manufacturing supports highly individualized devices that improve patient comfort and functionality while shortening production times compared with traditional methods. Beyond established players, innovative smaller firms and startups across Europe are working to reduce costs and expand access, including efforts to apply low-cost scanning and printing workflows in clinical settings.
The Asia Pacific region is likely to be the fastest-growing region in the 3D printed prosthetics market in 2026, driven by a combination of demographic, economic, and technological trends. Countries such as China and India are witnessing increased healthcare investments and growing demand for cost-effective, personalized prosthetic solutions due to rising incidences of amputations from trauma, diabetes, and traffic accidents. Governments in the region are actively promoting digital healthcare infrastructure and additive manufacturing technologies, recognizing their potential to improve access and reduce production costs for prosthetic devices, especially in rural and underserved areas. Hospitals and rehabilitation centers across metropolitan hubs are increasingly deploying 3D printing workflows to produce customized sockets and limb components with reduced lead times and improved fit.
Within this trend of rapid regional growth, companies are beginning to tailor their offerings and expand operations specifically for the Asia Pacific market, bridging technology gaps and improving market access. For example, Instalimb Corporation, a Japanese developer of AI-assisted 3D printed prosthetics, has been selected for the United Nations Industrial Development Organization (UNIDO) South Technology Transfer Program to help digitalize prosthetic manufacturing across India and South Asia, addressing supply shortages and improving quality through standardized digital workflows and local production partnerships. Instalimb’s initiative demonstrates how advanced additive manufacturing technology and localized implementation strategies can be leveraged to overcome traditional barriers such as cost, availability, and geographic distribution.
The global 3D printed prosthetics market exhibits a moderately fragmented structure, driven by a diverse mix of established medical technology companies, specialized additive manufacturing firms, and innovative startups competing across regions and applications. This fragmentation reflects the wide range of product offerings from customized sockets and limb components to advanced bionic designs and the ongoing integration of digital workflows such as 3D scanning, automated design software, and localized printing hubs to reduce lead times and costs.
With key leaders including 3D Systems Corporation, Stratasys Ltd., EnvisionTEC, YouBionic, UNYQ, Mecuris, and Open Bionics, companies are continually pushing the boundaries of prosthetic design and personalization to capture market share. These players compete through product innovation, such as developing biocompatible materials, multi-material printing capabilities, and enhanced functional performance, as well as strategic initiatives such as geographic expansion, localized manufacturing, and strategic collaborations. Market participants also increasingly pursue digital platforms that integrate remote scanning and cloud-based design to broaden reach and improve service offerings.
Key Industry Developments:
The global 3D printed prosthetics market is projected to reach US$2.0 billion in 2026.
Advancements in 3D printing technology, enabling customized, cost-effective, and lightweight prosthetic solutions, along with rising incidences of limb loss, are driving the 3D printed prosthetics market.
The 3D printed prosthetics market is expected to grow at a CAGR of 8.3% from 2026 to 2033.
Key market opportunities lie in expanding pediatric and personalized prosthetic solutions, localized point-of-care 3D printing, and growing adoption in emerging healthcare markets.
3D Systems Corporation, EnvisionTEC, Stratasys Ltd., Bionicohand, YouBionic, and Mecuris are the leading players.
| Report Attribute | Details |
|---|---|
| Historical Data | 2020 – 2025 |
| Forecast Period | 2026 – 2033 |
| Market Analysis | Value: US$ Bn |
| Geographical Coverage |
|
| Segmental Coverage |
|
| Competitive Analysis |
|
| Report Highlights |
|
By Product Type
By Material
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author