ID: PMRREP34677| 188 Pages | 27 Jan 2026 | Format: PDF, Excel, PPT* | Healthcare

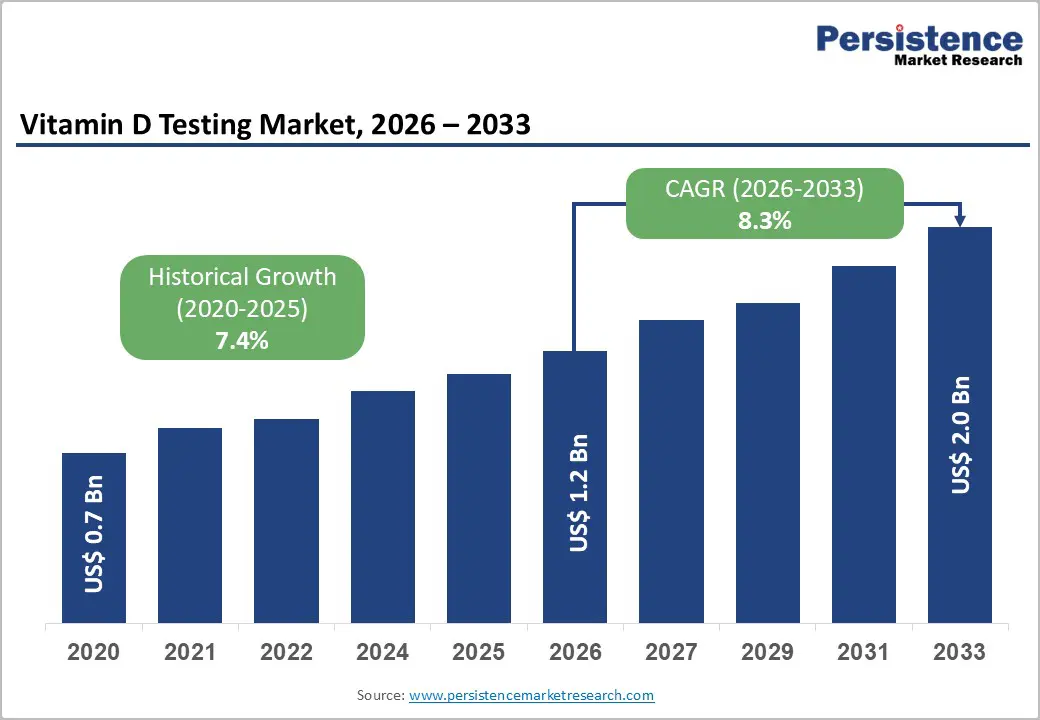
The global vitamin D testing market is estimated to grow from US$ 1.2 billion in 2026 to US$ 2.0 billion by 2033, at a CAGR of 8.3% over the forecast period.
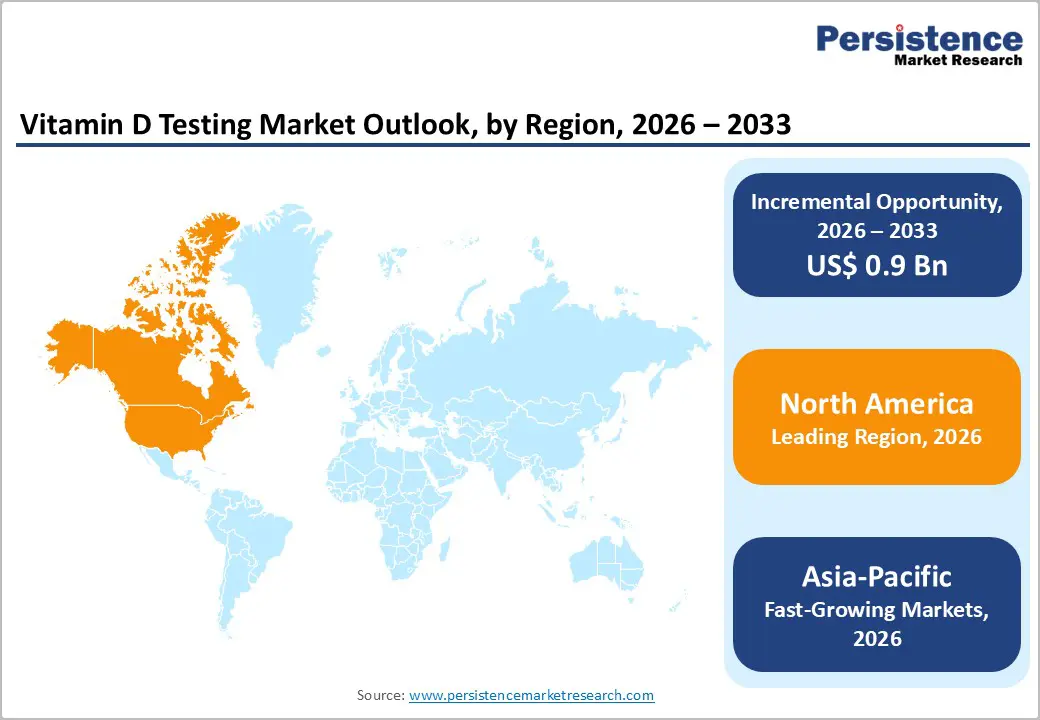
The global market is expanding steadily, driven by rising awareness of vitamin D deficiency, the adoption of preventive healthcare, and increased routine screening. North America dominates due to advanced diagnostic infrastructure and high testing volumes, while Asia-Pacific is the fastest-growing region, supported by expanding healthcare access, growing awareness, and improving laboratory capabilities.
| Key Insights | Details |
|---|---|
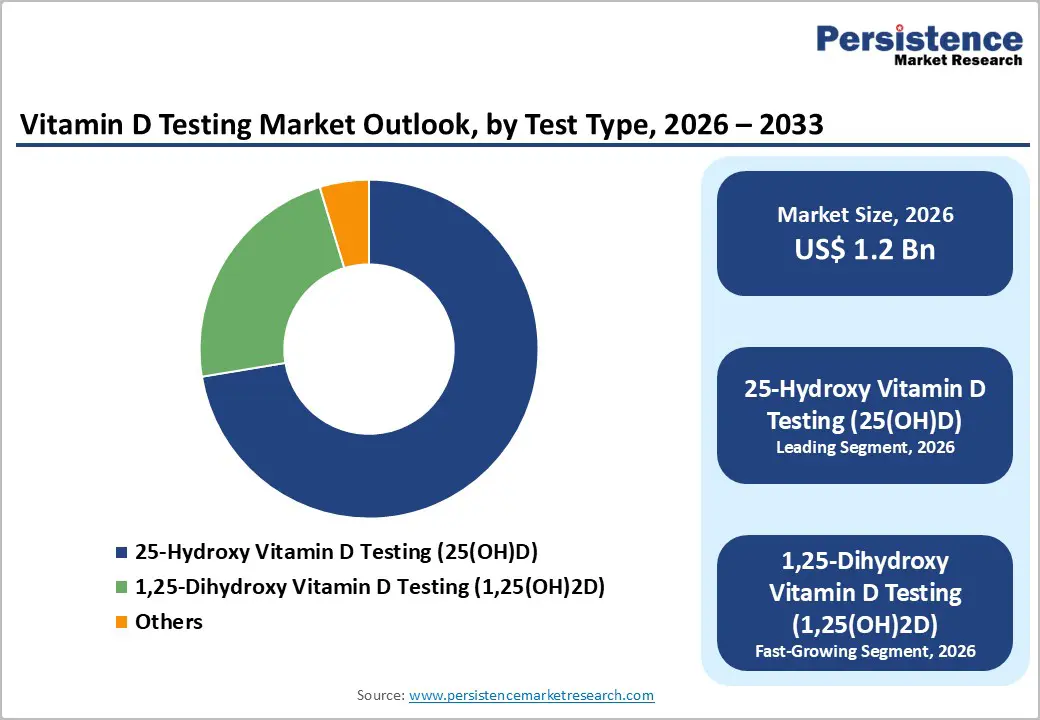
| Vitamin D Testing Market Size (2026E) | US$ 1.2 Bn |
| Market Value Forecast (2033F) | US$ 2.0 Bn |
| Projected Growth (CAGR 2026 to 2033) | 8.3% |
| Historical Market Growth (CAGR 2020 to 2025) | 7.4% |
The increased awareness and diagnosis of rare diseases have become pivotal drivers in the vitamin D testing market, significantly influencing demand for plasma-derived products. Rare diseases, often termed orphan diseases, affect a small percentage of the population.
However, collectively, they represent a substantial public health issue, with over 7,000 identified rare diseases impacting millions globally. Historically, the diagnosis and treatment of these conditions were hampered by a lack of awareness, limited diagnostic tools, and insufficient medical knowledge. This scenario has changed markedly in recent years, driven by multiple factors.
The advancements in diagnostic techniques have revolutionized the identification of rare diseases. Genetic testing, next-generation sequencing (NGS), and improved imaging technologies enable earlier and more accurate diagnosis. These sophisticated tools help identify genetic mutations and other biomarkers associated with rare diseases, enabling timely intervention and management.
Early diagnosis is crucial in managing rare diseases, as it can prevent complications, improve quality of life, and in some cases, offer curative treatments. Moreover, heightened awareness among healthcare professionals and the public plays a significant role. Initiatives by healthcare organizations, patient advocacy groups, and government agencies have been instrumental in spreading awareness of rare diseases.
Seasonal variations play a crucial role as sunlight exposure, a primary source of vitamin D synthesis in the skin, fluctuates throughout the year. Regions farther from the equator experience more significant seasonal variations in sunlight intensity and duration.
For instance, individuals in northern latitudes may have lower vitamin D levels during winter months due to reduced sunlight exposure, potentially leading to seasonal fluctuations in test results. Conversely, those in equatorial regions might exhibit more stable levels year-round.
Geographic location also impacts vitamin D synthesis. People living in urban areas with tall buildings or high levels of pollution may receive less sunlight, which can affect their vitamin D production compared to those in rural or less polluted areas.
Additionally, cultural practices and lifestyle choices, such as religious attire that covers much of the body, can further reduce sunlight exposure, influencing vitamin D levels irrespective of geographical location. Individual differences in metabolism contribute to the complexity of interpretation. Factors such as age, skin pigmentation, body mass index (BMI), and underlying health conditions can affect how efficiently the body synthesizes and utilizes vitamin D.
For instance, older adults and individuals with darker skin tones may require more sunlight exposure to produce adequate vitamin D compared to younger individuals with lighter skin tones.
Moreover, genetic variations in vitamin D receptor genes and enzymes involved in its metabolism can influence individual responses to vitamin D intake and impact test results. Such complexities underscore the need for personalized approaches to interpreting vitamin D levels, accounting for a person’s unique circumstances and health history.
The increased incidence of chronic diseases linked to vitamin D deficiency, such as osteoporosis and cardiovascular diseases, underscores the critical need for regular testing and monitoring of vitamin D levels.
Osteoporosis, a condition characterized by weakened bones prone to fractures, has been closely associated with insufficient vitamin D levels. Vitamin D plays a crucial role in calcium absorption and bone metabolism, making adequate levels essential for maintaining bone health and density.
As the population ages and lifestyles shift towards more sedentary indoor activities, the risk of osteoporosis rises, further emphasizing the importance of proactive testing to identify and mitigate deficiencies early on. Cardiovascular diseases, including hypertension, coronary artery disease, and heart failure, have also been linked to vitamin D deficiency through various mechanisms.
Vitamin D receptors are present in the cardiovascular system, influencing processes such as regulation of vascular tone, modulation of inflammation, and endothelial function.
Deficiencies in vitamin D may contribute to endothelial dysfunction, arterial stiffness, and increased systemic inflammation, all of which are risk factors for cardiovascular conditions. Given the widespread prevalence of cardiovascular diseases globally and their significant impact on public health, regular monitoring of vitamin D levels can support early intervention and management.
The need for regular vitamin D testing is further amplified by evolving understandings of its role beyond skeletal and cardiovascular health. Research continues to uncover associations between vitamin D deficiency and increased risks of autoimmune diseases, certain cancers, and even mental health disorders.
Such findings highlight the broad-reaching implications of maintaining optimal vitamin D levels throughout life. By integrating routine testing into healthcare protocols, healthcare providers can better tailor supplementation regimens and lifestyle recommendations to mitigate the risks associated with deficiency, ultimately promoting better overall health outcomes for individuals at risk of chronic diseases linked to vitamin D insufficiency.
25-Hydroxy Vitamin D Testing (25(OH)D) holds a 72.4% share of the global market in 2025, as it is the most reliable and widely accepted biomarker for assessing overall vitamin D status in clinical practice. Serum 25(OH)D reflects both dietary intake and cutaneous synthesis and has a half-life of approximately two to three weeks, making it a stable indicator of body vitamin D reserves compared with the active form, 1,25(OH) D, which circulates at much lower concentrations and does not correlate with total vitamin D status. Clinical guidelines from endocrine and public health authorities recommend serum 25(OH)D measurement to evaluate deficiency, defined as levels below established cut-offs (e.g., <20 ng/mL), because approximately 19-73 percent of populations surveyed in national studies have insufficient or deficient levels, underscoring its diagnostic utility.
Immunoassays dominate the vitamin D testing market primarily because they are automated, rapid, and compatible with high-throughput clinical laboratory workflows, enabling widespread screening of 25-hydroxyvitamin D across large patient volumes. These methods (e.g., CLIA, ELISA) deliver results faster and with simpler operations than chromatographic techniques, which require specialized instrumentation and longer processing times. Although mass spectrometry (LC-MS/MS) is the reference method for accuracy, immunoassays remain predominant in clinical practice due to their integration into routine hospital and diagnostic center analyzers. National standardization programs, such as the CDC’s Vitamin D Standardization-Certification Program, certify immunoassays that meet stringent accuracy and precision criteria, further reinforcing their adoption in clinical settings.
North America dominates the vitamin D testing market with a 41.7% share in 2025, driven by a high prevalence of deficiency and a well-established healthcare system that prioritizes preventive diagnostics. National surveys in the United States have shown that vitamin D deficiency affects a substantial portion of the population, with earlier NHANES data indicating that up to around 18 percent of adults are at risk of deficiency or inadequacy when defined by low serum 25(OH)D levels, prompting regular clinical screening. The U.S. and Canada maintain advanced laboratory infrastructure and routine inclusion of vitamin D testing in preventive care protocols, and widespread insurance coverage further facilitates access to diagnostic services. This combination of health burden, clinical emphasis, and system capacity underpins North America’s leading share of the global market.
Europe is an important region in the vitamin-d testing market because vitamin-D deficiency is highly prevalent across many European populations, making screening crucial for public health. Large population studies show that in Europe, about 18% of individuals have serum 25(OH)D levels below 30 nmol/L, and approximately 53% are below 50 nmol/L, indicating widespread insufficiency and deficiency that warrant clinical testing and monitoring. European countries also include high-risk groups such as postmenopausal women, where studies report up to 79.6% prevalence of 25(OH)D inadequacy using common cut-offs, especially in older age groups. This high disease burden, combined with established healthcare systems and preventive screening practices across EU nations, underscores the region’s role as a key market for routine and standardized vitamin-D testing.
Asia-Pacific is the fastest-growing region in the vitamin D testing market because rapid digital Asia Pacific is the fastest-growing region in the Vitamin D Testing Market because of the extremely high prevalence of deficiency and expanding healthcare access. Meta-analyses show that in South Asian countries, pooled vitamin-D deficiency prevalence reaches approximately 68%, with rates as high as 67% in India and Pakistan, 67% in Bangladesh, and 57% in Nepal, indicating widespread deficiency across large populations and driving testing demand. Urbanization, indoor lifestyles, air pollution, and cultural practices that limit sun exposure further contribute to low vitamin D levels, increasing the need for routine screening. These health trends, coupled with improving laboratory infrastructure and growing awareness of the consequences of deficiencies, underpin the rapid uptake of vitamin D testing in the Asia Pacific.
Leading vitamin D testing market companies focus on advanced diagnostics, AI-driven analytics, and telehealth integration. Emphasis on interoperability, accuracy, and user-friendly platforms supports preventive care and patient engagement. Strategic collaborations with healthcare providers and regulatory bodies accelerate adoption, innovation, and global integration, driving growth in routine screening, digital health, and expanded clinical and remote monitoring solutions.
The global vitamin D testing market is projected to be valued at US$ 1.2 Bn in 2026.
Rising deficiency prevalence, preventive healthcare adoption, aging populations, routine screening, and growing awareness drive market growth.
The global vitamin D testing market is poised to witness a CAGR of 8.3% between 2026 and 2033.
Expansion of routine screening, advanced testing techniques, emerging economies, telehealth integration, and personalized preventive healthcare opportunities.
Johnson & Johnson Services, Inc., Thermo Fisher Scientific, Inc., DexCom, Inc., Siemens Healthineers, Abbott Laboratories, Metrohm AG.
| Report Attribute | Details |
|---|---|
| Historical Data/Actuals | 2020 - 2025 |
| Forecast Period | 2026 - 2033 |
| Market Analysis | Value: US$ Bn |
| Geographical Coverage |
|
| Segmental Coverage |
|
| Competitive Analysis |
|
| Report Highlights |
|
By Test Type
By Technique
By Indiction
By End-user
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author