ID: PMRREP34618| 200 Pages | 11 Feb 2026 | Format: PDF, Excel, PPT* | Healthcare

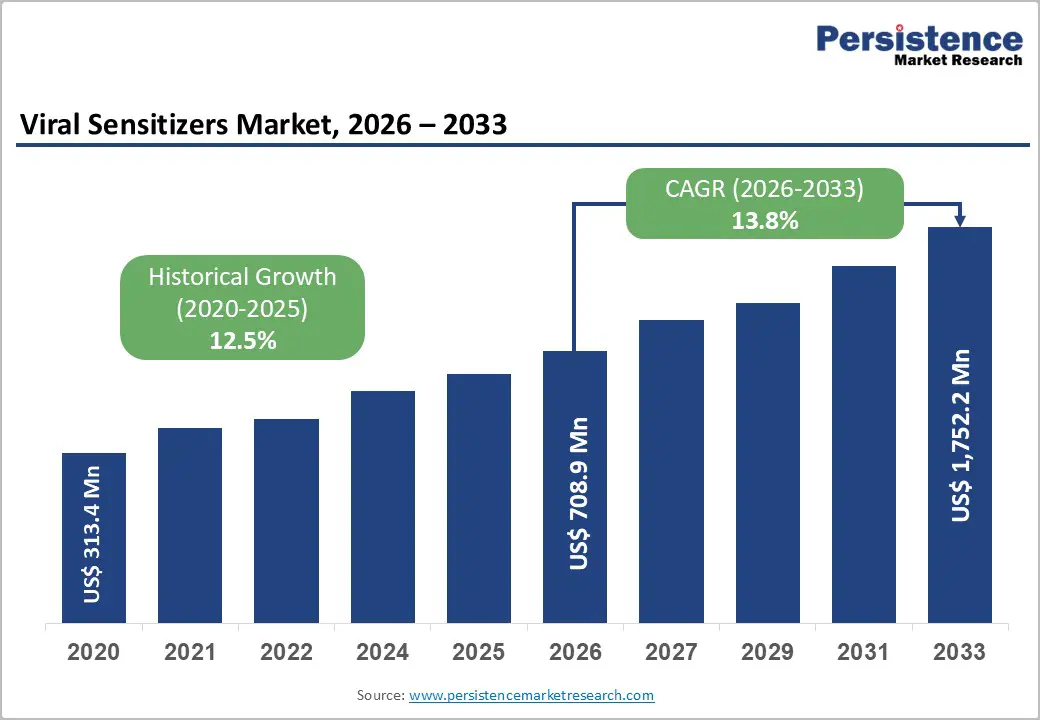
The global viral sensitizers market is estimated to grow from US$ 708.9 million in 2026 to US$ 1,752.2 million by 2033, growing at a CAGR of 13.8% from 2026 to 2033.
The global viral sensitizers market is growing steadily, driven by rising demand for antiviral therapies, the adoption of digital healthcare, and advances in analytics. North America leads with strong infrastructure, strict regulatory oversight, and high-quality production standards. Asia-Pacific is the fastest-growing region, supported by expanding healthcare facilities, government initiatives, rising patient awareness, and investments in interoperable diagnostic and manufacturing solutions.
| Key Insights | Details |
|---|---|
|
Global Viral Sensitizers Market Size (2026E) |
US$ 708.9 Mn |
|
Market Value Forecast (2033F) |
US$ 1,752.2 Mn |
|
Projected Growth (CAGR 2026 to 2033) |
13.8% |
|
Historical Market Growth (CAGR 2020 to 2025) |
12.5% |
The global burden of viral diseases remains high, sustaining demand for antiviral and immune-modulating treatments that often rely on viral sensitizers to improve therapeutic efficacy. According to the World Health Organization (WHO), nearly 39 million people were living with HIV in 2022, while hundreds of millions are chronically infected with hepatitis B or C, creating a persistent need for advanced therapies. Viral infections also contribute significantly to annual morbidity and mortality worldwide, with seasonal influenza alone causing 3–5 million severe cases each year, per WHO estimates.
Moreover, public health initiatives have expanded access to antivirals and improved treatment adherence, thereby increasing the use of adjunct technologies that enhance drug performance, including immunomodulatory sensitizers. Growth in antiviral prescriptions and clinical trials further underscores expanded therapeutic demand, reinforcing the role of sensitizers in supporting targeted immune responses and improving clinical outcomes.
Manufacturing complex biologic and viral-targeted agents, which often incorporate multiple viral sensitizers, entails substantial costs and technical requirements. The Tufts Center for Drug Development reports the average cost to develop a new therapeutic remains near $897 million, with biologically based products often exceeding this due to sophisticated production and stringent quality demands. Such high R&D and manufacturing expenses must be recouped through pricing, limiting access and adoption, particularly in resource-limited settings.
In addition, specialized viral vector and cell-based manufacturing platforms are expensive: a typical viral vector gene therapy manufacturing run can cost close to $100 000 per dose at current scales, according to pharmaceutical engineering analyses, largely due to raw material inputs and low yields. These cost burdens constrain clinical adoption of advanced sensitizers and can deter smaller developers from investing in novel platforms.
The evolution of synthetic and next-generation viral sensitizers presents a strong opportunity to meet clinical and manufacturing needs more efficiently. Advances in biologics and platform technologies, such as automated continuous manufacturing, are expanding capabilities to produce complex agents with greater consistency and scalability, addressing historic bottlenecks in biologic outputs. Continued innovation supported by increased R&D expenditure in virology and immunology enhances prospects for tailored sensitizers with improved safety and specificity.
Furthermore, digital tools and computational biology are enabling rational design of sensitizers that target viral pathways more effectively, with AI-aided optimization reducing experimental cycles and potentially lowering development timelines. These advances enable broader integration of synthetic sensitizers into personalized antiviral regimens and combination therapies across infectious and immune-mediated diseases.
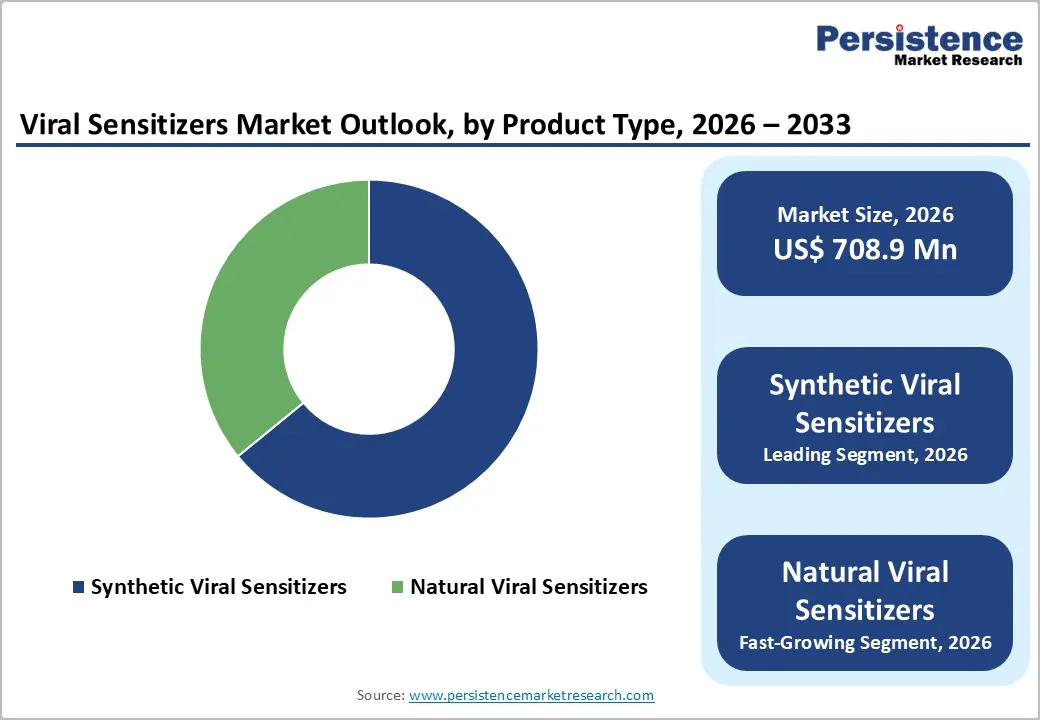
Synthetic viral sensitizers occupies 64.1% share of the global market in 2025, because they offer engineered precision, reproducibility, and scalability compared with naturally derived alternatives. These sensitizers can be tailored for specific viral pathways, targeted immune modulation, and reduced off-target effects, improving therapeutic efficacy and safety. Their production is compatible with advanced biomanufacturing platforms, allowing consistent quality, large-scale output, and compliance with stringent regulatory standards. Furthermore, synthetic sensitizers integrate with digital monitoring and AI-driven optimization, enabling real-time assessment of patient response, dosage, and therapy effectiveness. Global adoption is increasing in antiviral therapies, immunomodulation, and personalized medicine, where controlled activity and predictable performance are critical for clinical success and large-scale deployment in hospitals and research facilities.
Antiviral drug development dominates the market as viral infections remain a significant global health burden, necessitating constant innovation in therapeutics. Viral sensitizers enhance drug performance by improving viral targeting, boosting potency, and modulating immune responses, making them essential in developing effective antiviral agents. WHO reports approximately 254 million people with hepatitis B and 50 million with hepatitis C, while seasonal influenza affects millions annually, highlighting ongoing demand. Sensitizers are critical during preclinical and clinical development for screening, efficacy optimization, and combination therapy strategies. This focus drives pharmaceutical R&D, accelerating novel antiviral discovery and enabling safer, more potent therapies to treat emerging and chronic viral diseases worldwide.
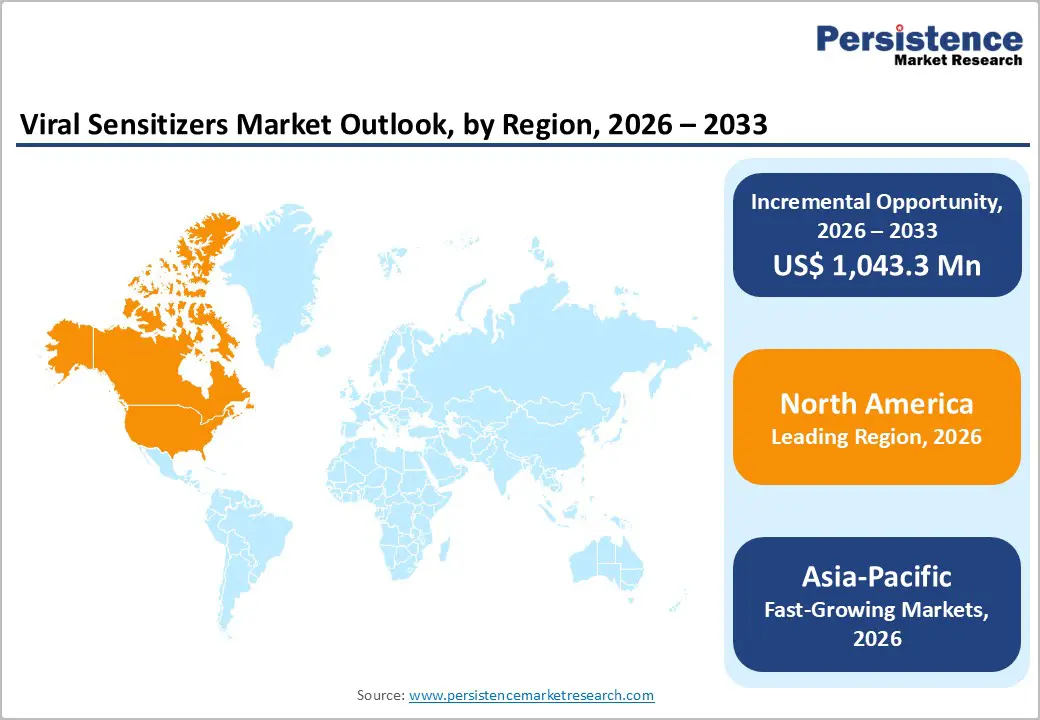
North America dominates the viral sensitizers market with 38.9% share in 2025, due to its advanced biopharmaceutical and healthcare ecosystem, strong R&D investment, and robust regulatory infrastructure. The region contributed roughly 38–40 % of the infectious disease therapeutics market, reflecting deep capabilities in developing and deploying novel antiviral agents and adjunct technologies. Higher healthcare spending and infrastructure enable rapid adoption of innovative modalities that enhance antiviral performance. Government and private funding support extensive clinical trial activity and early adoption of complex therapies, including those using viral sensitizers. The substantial concentration of biotech companies, research institutions, and venture capital further accelerates innovation. This depth of scientific and commercial capability ensures North America retains leadership in emerging viral technologies and therapeutic enhancements.
Europe is an important region for viral sensitizers because of its coordinated public health infrastructure, strong regulatory frameworks, and collaborative healthcare policies. Agencies like the European Centre for Disease Prevention and Control (ECDC) and EU health programs such as EU4Health (€5.1 billion budget for 2021–2027) enhance disease surveillance, preparedness, and therapeutic development. These platforms support scientific collaboration and data sharing across EU member states, strengthening readiness for viral outbreaks and fostering innovation in therapeutics and adjunct technologies. Europe’s integrated health systems facilitate clinical adoption of advanced antiviral treatments and adjunct sensitizing agents within standardized care pathways. The region’s commitment to real-world evidence (RWE) and regulatory harmonization helps streamline the evaluation of novel therapies. This environment ensures Europe remains strategically significant for commercial and clinical deployment of viral sensitization technologies.
Asia Pacific is the fastest-growing market for viral sensitizers due to expanding healthcare infrastructure, increasing government investment, and rising infectious disease burden across populous countries. The region has experienced dramatic growth in biopharmaceutical innovation and clinical activity, with Asia’s share of the global innovative drug pipeline increasing from 28% to 43% over five years, outpacing both the United States and Europe. Rapid expansion of domestic R&D, manufacturing capacities, and clinical research activities, particularly in China and South Korea drives adoption of advanced therapeutic technologies, including sensitizers. Population factors also contribute: Asia-Pacific remains home to large segments affected by infectious diseases that require ongoing innovations in treatment and supportive technologies. Combined with supportive policies and improving healthcare access, these dynamics fuel the region’s rapid growth trajectory.
Leading viral sensitizers companies focus on advanced antiviral and immunomodulatory technologies, scalable production, and regulatory compliance. Investments target process optimization, AI-driven design, and high-throughput testing to ensure efficacy and consistency. Strategic collaborations with biotech, academia, and regulators, coupled with stringent quality control and integrated supply chains, accelerate global adoption of viral sensitizers in therapeutic and research applications.
The global viral sensitizers market is projected to be valued at US$ 708.9 Mn in 2026.
Rising viral infections, demand for antiviral therapies, technological advances, and expanding healthcare infrastructure drive market growth.
The global viral sensitizers market is poised to witness a CAGR of 13.8% between 2026 and 2033.
Opportunities include synthetic viral sensitizers, next-generation therapies, AI optimization, scalable manufacturing, and emerging biotech markets.
Moderna, Pfizer, AbbVie, Bluebird Bio, Merck & Co., and Iovance Biotherapeutics.
| Report Attribute | Details |
|---|---|
|
Historical Data/Actuals |
2020 - 2025 |
|
Forecast Period |
2026 - 2033 |
|
Market Analysis |
Value: US$ Mn |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
By Product Type
By Application
By End-user
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author