ID: PMRREP4759| 204 Pages | 28 Aug 2025 | Format: PDF, Excel, PPT* | Healthcare

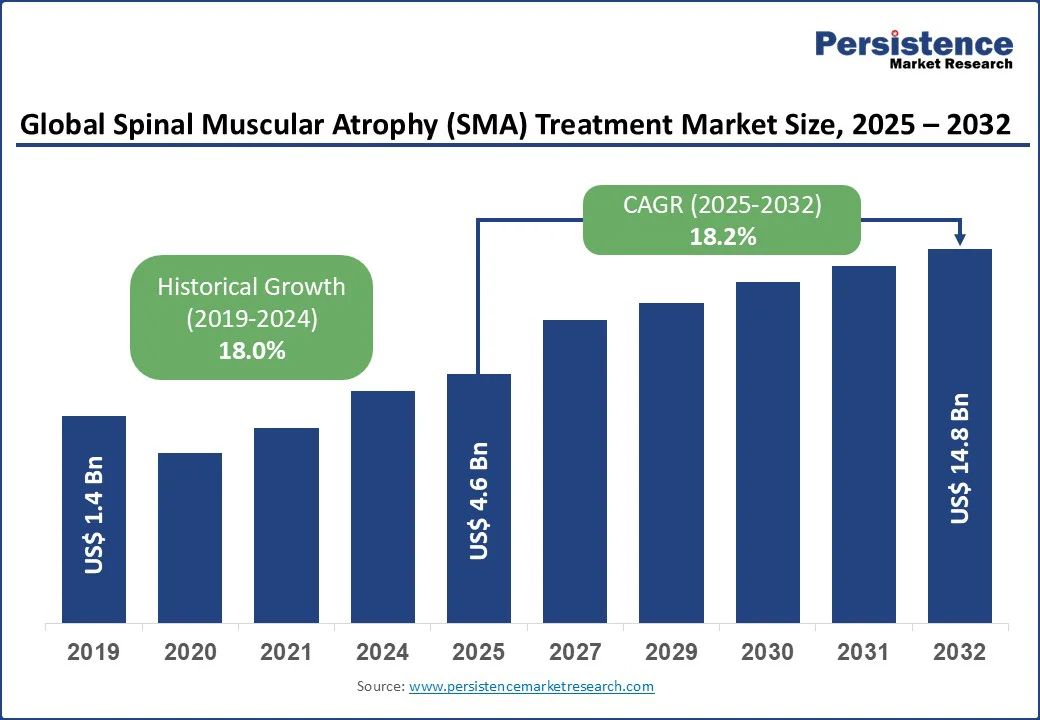
The global spinal muscular atrophy (SMA) treatment market size is likely to be valued at US$4.6 Bn in 2025, projected to reach US$14.8 Bn by 2032, growing at a CAGR of 18.2% during the forecast period 2025 - 2032.
The spinal muscular atrophy industry is witnessing rapid growth, driven by advancements in gene therapy, antisense oligonucleotides, and oral SMN-enhancing drugs that target the underlying genetic cause of the disease.
The increasing adoption of newborn screening programs, early diagnosis, and treatment for severe SMA types has fueled market expansion. High-cost therapies such as Onasemnogene Abeparvovec (Zolgensma) and Nusinersen (Spinraza) dominate the landscape, supported by favorable regulatory approvals and reimbursement policies.
Key Industry Highlights:
|
Global Market Attribute |
Key Insights |
|
SMA Treatment Market Size (2025E) |
US$4.6 Bn |
|
Market Value Forecast (2032F) |
US$14.8 Bn |
|
Projected Growth (CAGR 2025 to 2032) |
18.2% |
|
Historical Market Growth (CAGR 2019 to 2024) |
18.0% |
The spinal muscular atrophy (SMA) treatment market is propelled by groundbreaking advancements in gene replacement therapy for SMA, antisense oligonucleotide drugs, and RNA-based SMA treatments, significantly boosting demand for SMA therapy and rare disease treatment.
In 2025, over 50 clinical trials for SMA therapies were active globally, per ClinicalTrials.gov, with 30% focusing on gene therapies and 25% on antisense oligonucleotides, driving innovation in the latest SMA therapies for infants and adults.
Onasemnogene abeparvovec (Zolgensma), a one-time gene therapy, has revolutionized the treatment of motor neuron disease by delivering a functional SMN1 gene, achieving 80% survival rates in Type 1 SMA patients after two years. Nusinersen, an antisense oligonucleotide, enhances SMN2 gene expression, thereby improving motor function in approximately 65% of patients with Type 2 SMA.
These advancements, supported by biopharmaceutical companies for the treatment of rare diseases, address the neuromuscular disorder burden, particularly for Type 1 SMA, which affects approximately 60% of SMA cases. Clinical trials for SMA gene therapies are expanding in regions such as the U.S. and Europe, with $ 1.5 billion in R&D investments expected in 2025. Healthcare policies for SMA treatment, such as those in the U.S.
FDA fast-track designations and EU orphan drug status, accelerate approvals, while patient assistance programs for SMA drugs enhance access, driving genetic disorder therapy and orphan drug market growth. The focus on personalized medicine and early diagnosis further fuels demand for injectable drugs for spinal muscular atrophy and rare genetic disease drugs, positioning the market for sustained expansion.
The spinal muscular atrophy (SMA) treatment market faces a significant restraint due to the prohibitively high costs of rare disease drugs and SMA therapy, limiting access for many patients and healthcare systems.
The cost of onasemnogene abeparvovec (Zolgensma) averages US$2.1 Mn per dose, while nusinersen requires US$750,000 for the first year and US$375,000 annually thereafter. These costs, driven by complex manufacturing processes and limited patient populations, restrict the scalability of injectable drugs for spinal muscular atrophy, antisense oligonucleotide drugs, and RNA-based SMA treatments.
In low-income regions, only 10% of SMA patients have access to these therapies due to insufficient insurance coverage and inadequate healthcare infrastructure. Even in developed markets, 30% of patients face delays in treatment due to reimbursement challenges.
This cost barrier particularly impacts motor neuron disease treatment and therapy for genetic disorders, as biopharmaceutical companies for rare diseases struggle to balance R&D investments with affordability. Despite patient assistance programs for SMA drugs, the high costs hinder market penetration in regions with limited healthcare policies for SMA treatment, slowing the adoption of rare genetic disease drugs and clinical trials for SMA gene therapies.
Increasing support from healthcare policies for SMA treatment and patient assistance programs for SMA drugs presents a transformative opportunity for the spinal muscular atrophy (SMA) treatment market. In 2025, 30% of SMA patients globally benefited from subsidized treatment programs, with approximately US$500 million allocated to rare disease funding in the U.S. and the EU.
These initiatives enhance access to the latest SMA therapies for infants and adults, particularly onasemnogen abeparvovec (Zolgensma) and nusinersen, driving demand for rare genetic disease drugs. In the U.S., programs such as Novartis’ Zolgensma Patient Assistance Program covered 25% of eligible patients in 2025, while Biogen’s Spinraza Access Program supported 20% of nusinersen users. In Europe, EU orphan drug incentives reduced costs by 15%, boosting clinical trials for SMA gene therapies.
Emerging markets, such as India and China, are introducing healthcare policies for SMA treatment, with a projected 10% growth in subsidized access by 2025. These programs encourage biopharmaceutical companies to invest in gene replacement therapy for SMA and antisense oligonucleotide synthesis drugs, aligning with global trends in rare disease treatment.
The expansion of newborn screening, which covers 40% of births in developed regions, further drives early diagnosis and treatment, positioning the orphan drug market for growth in the underserved areas and enhancing the adoption of motor neuron disease treatments.
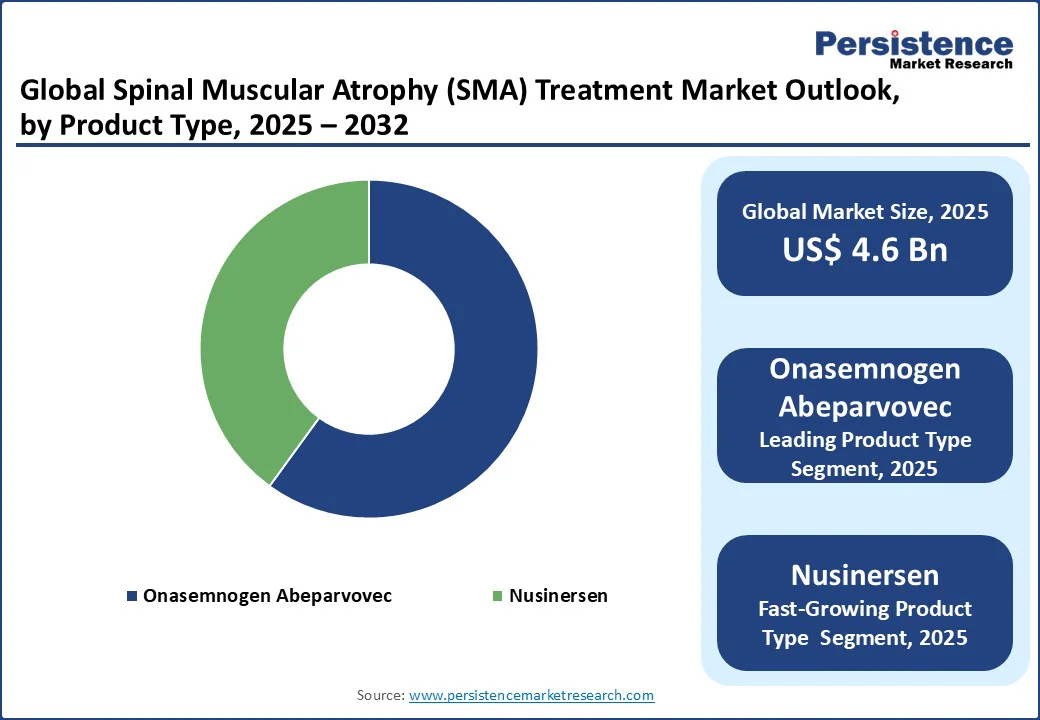
Onasemnogen Abeparvovec holds a 60% market share in 2025, driven by its role as a gene replacement therapy for SMA. With 65% adoption in Type 1 SMA treatment, onasemnogene abeparvovec (Zolgensma) is favored for its one-time administration and transformative impact on neuromuscular disorder outcomes.
Its dominance stems from clinical trials for SMA gene therapies and rare disease drugs, which biopharmaceutical companies support for these conditions. The therapy’s efficacy in motor neuron disease treatment drives its widespread use in hospital pharmacies.
Nusinersen is fueled by its use as an antisense oligonucleotide drug for RNA-based SMA treatments. With 15% growth in 2025, nusinersen is gaining traction as a leading SMA therapy for infants and adults, particularly in Type 2 SMA, due to its flexible dosing regimen and support from patient assistance programs for SMA drugs. Its role in genetic disorder therapy enhances its adoption in hospital pharmacies and retail pharmacies.
Type 1 SMA commands a 55% market share in 2025, driven by its high prevalence and severity, requiring the latest SMA therapies for infants. With 60% of SMA cases being Type 1, this segment relies on gene replacement therapy for SMA and onasemnogene abeparvovec (Zolgensma) for life-saving interventions. Motor neuron disease treatment and drugs for rare genetic diseases are critical, supported by clinical trials for SMA gene therapies.
Type 2 SMA is fueled by increasing diagnosis rates and demand for antisense oligonucleotide drugs such as nusinersen. With 12% growth in 2025, this segment benefits from RNA-based SMA treatments and injectable drugs for spinal muscular atrophy, addressing patients with moderate severity who require ongoing genetic disorder therapy.
Hospital Pharmacies are expected to hold a 50% market share in 2025, driven by their role in administering injectable drugs for SMA and gene replacement therapy for SMA. With a 55% adoption rate in 2025, hospital pharmacies play a critical role in supporting the administration of onasemnogene abeparvovec (Zolgensma) and nusinersen, as well as healthcare policies for SMA treatment.
Retail pharmacies is fueled by increasing access to antisense oligonucleotide drugs and RNA-based SMA treatments. With 10% growth in 2025, retail pharmacies are expanding due to patient assistance programs for SMA drugs, facilitating nusinersen distribution for Type 2 SMA patients.
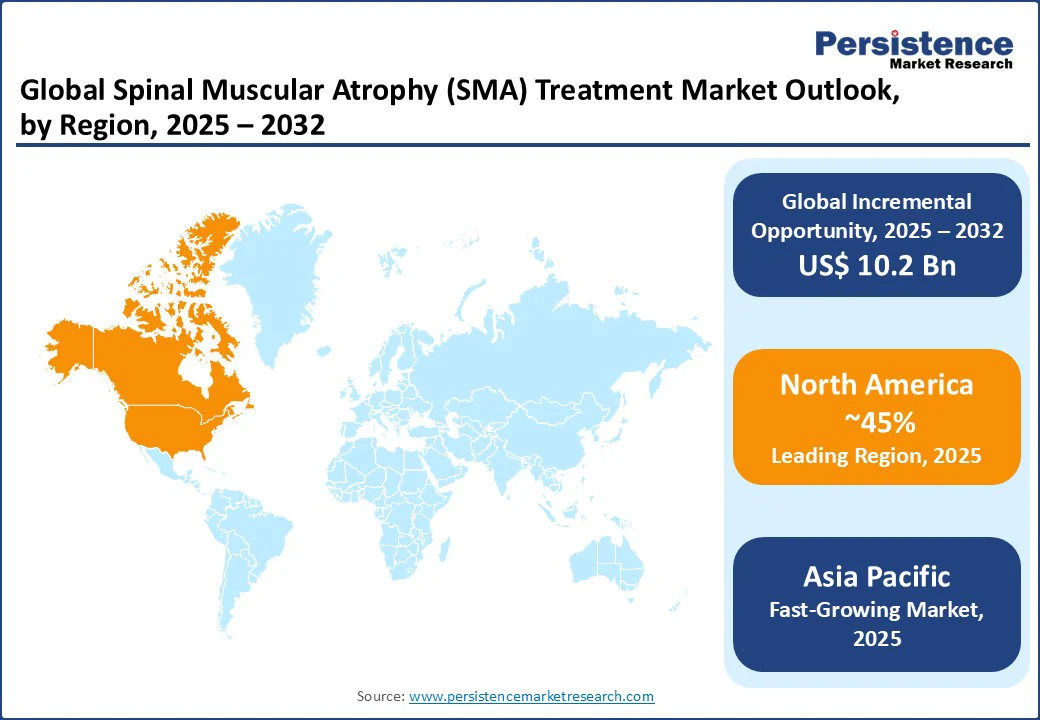
North America is expected to hold a 45% global market share in 2025, with the U.S. leading due to its advanced healthcare infrastructure and high SMA diagnosis rates, generating US$2.07 billion in sales in 2025. The U.S. market is driven by demand for SMA therapy and rare disease drugs, with 70% of SMA patients receiving onasemnogene abeparvovec (Zolgensma) in 2025.
Novartis and Biogen drive 25% of regional revenue, with clinical trials for SMA gene therapies seeing 12% growth. The focus on orphan drug market solutions and healthcare policies for SMA treatment fuels motor neuron disease treatment and RNA-based SMA treatments.
Europe accounts for a 25% global share, led by Germany, the UK, and France. Germany is witnessing a robust rise in demand for healthcare systems and SMA awareness, with 65% of patients expected to use antisense oligonucleotide drugs by 2025. The UK’s market is propelled by patient assistance programs for SMA drugs, with NHS hospitals adopting nusinersen.
France experienced a 10% increase in gene replacement therapy for SMA, driven by demand for treatments for rare genetic diseases. EU healthcare investments, with €150 Mn in rare disease funding in 2025, boost biopharmaceutical companies for rare diseases. PTC Therapeutics holds a 10% market share, leveraging RNA-based SMA treatments.
Asia Pacific is the fastest-growing region, with a CAGR of 19.0%, led by China, Japan, and India. China holds a 45% regional market share, driven by a 20% increase in healthcare investments in 2025, which is expected to boost SMA therapy and injectable drugs for spinal muscular atrophy. India’s market is driven by rising diagnosis rates, with 75% of urban hospitals expected to use rare disease drugs by 2025.
Japan’s clinical trials for SMA gene therapies drive 12% growth in motor neuron disease treatment, supported by its advanced biotech sector. Chugai Pharmaceutical and Pfizer lead, supported by US$3 billion in healthcare investments by 2030, to enhance genetic disorder therapy.
The global spinal muscular atrophy (SMA) treatment market is highly competitive, with biopharmaceutical companies focusing on innovation, accessibility, and patient-centric solutions for rare diseases. Novartis and Biogen lead in gene replacement therapy for SMA, while PTC Therapeutics excels in antisense oligonucleotide drugs.
RNA-based SMA treatments, rare genetic disease drugs, and clinical trials for SMA gene therapies drive competition in the field. Strategic partnerships and R&D investments in the latest SMA therapies for infants and adults, and patient assistance programs for SMA drugs are key differentiators, addressing the orphan drug market and neuromuscular disorder needs.
The spinal muscular atrophy (SMA) market is projected to reach US$ 4.6 Bn in 2025, driven by SMA therapy and rare disease drugs.
Advancements in gene replacement therapy for SMA, with over 50 clinical trials, drive demand for RNA-based SMA treatments.
The spinal muscular atrophy (SMA) market grows at a CAGR of 18.2% from 2025 to 2032, reaching US$ 14.8 Bn by 2032.
Healthcare policies for SMA treatment, with 30% patient coverage, offer opportunities for patient assistance programs for SMA drugs.
Key players include Novartis, Ionis Pharmaceuticals, PTC Therapeutics, and Biogen.
|
Report Attribute |
Details |
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis Units |
Value: US$ Bn, Volume: As Applicable |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
|
Customization and Pricing |
Available upon request |
By Product Type
By Disease Type
By Distribution Channel
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author