ID: PMRREP15376| 219 Pages | 30 Jan 2026 | Format: PDF, Excel, PPT* | Healthcare

The global spinal stenosis implant market size is estimated to grow from US$ 10.1 Bn in 2026 to US$ 16.6 Bn by 2033. The market is projected to record a CAGR of 5.3% during the forecast period from 2026 to 2033.
Global demand for spinal stenosis implants is increasing steadily, driven by the rising prevalence of degenerative spine disorders affecting the cervical, lumbar, and thoracic regions. Aging populations, longer life expectancy, sedentary lifestyles, and increasing incidence of osteoarthritis, disc degeneration, and spinal instability are expanding the patient pool requiring surgical intervention. Spinal stenosis implants are widely used across hospitals, specialty spine centers, and ambulatory surgical facilities to restore spinal stability, relieve neural compression, and improve functional mobility through both open and minimally invasive procedures. Growing preference for surgical solutions that offer durable pain relief, improved neurological outcomes, and faster recovery is accelerating adoption. Increased awareness of early diagnosis, improved access to advanced imaging, and rising acceptance of minimally invasive spine surgery further support demand. Technological advancements such as improved biomaterials, enhanced fixation systems, motion-preservation designs, and navigation-assisted implantation are improving clinical outcomes and procedural efficiency. Additionally, strengthening healthcare infrastructure in emerging markets and rising investments in orthopedic and spine care services are reinforcing long-term global demand for spinal stenosis implants.
| Key Insights | Details |
|---|---|
|
Spinal Stenosis Implant Market Size (2026E) |
US$ 10.1 Bn |
|
Market Value Forecast (2033F) |
US$ 16.6 Bn |
|
Projected Growth (CAGR 2026 to 2033) |
5.3% |
|
Historical Market Growth (CAGR 2020 to 2025) |
4.2% |
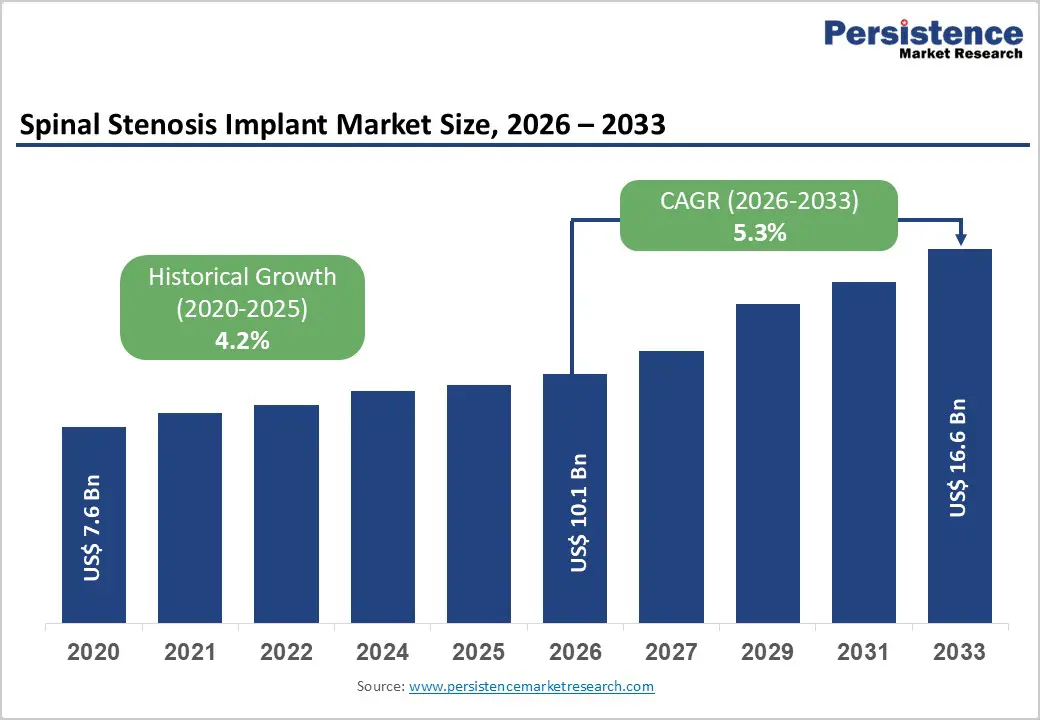
Market growth is strongly supported by the rising global incidence of degenerative spinal conditions, particularly spinal stenosis, driven by aging populations and longer life expectancy. Age-related disc degeneration, facet joint hypertrophy, and ligament thickening significantly increase the prevalence of cervical and lumbar stenosis, expanding the addressable patient pool requiring surgical intervention. As symptoms progress from chronic back pain to neurological impairment, implant-based procedures become a preferred treatment option to restore spinal stability and decompress neural structures.
Advancements in spinal implant technology, including improved fusion systems and motion-preservation devices, have enhanced surgical precision and clinical outcomes, encouraging wider adoption among spine surgeons. Growing acceptance of minimally invasive spine surgery further accelerates demand, as these procedures reduce hospital stays, blood loss, and recovery time. Increasing awareness among patients regarding the benefits of early surgical management is also contributing to higher procedure volumes. In parallel, expanding access to advanced diagnostic imaging has improved detection rates, particularly in developed healthcare systems. Collectively, these factors are driving sustained growth in implant utilization across hospitals and specialized spine centers worldwide.
The market expansion is constrained by the high cost associated with spinal implant procedures and related surgical care. Implant pricing, combined with operating room expenses, post-operative rehabilitation, and extended recovery periods, can limit affordability, particularly in cost-sensitive and underinsured populations. Surgical risks such as infection, implant failure, adjacent segment degeneration, and revision surgery concerns may also affect patient and physician decision-making.
Variability in surgical outcomes based on surgeon expertise and hospital infrastructure further impacts adoption, especially in regions with limited access to specialized spine surgeons. In low- and middle-income countries, shortages of trained professionals and advanced surgical facilities restrict procedural volumes. Additionally, stringent regulatory requirements for implant approval, clinical validation, and material compliance increase development costs and time-to-market for manufacturers. Conservative treatment approaches, including physical therapy and pain management, may delay or reduce surgical intervention in early-stage patients. Together, these challenges can slow market penetration and limit growth potential in certain regions and patient segments.
Significant growth opportunities are emerging from the rapid adoption of minimally invasive spine surgery techniques and expanding healthcare infrastructure in emerging economies. Surgeons increasingly favor implants compatible with less invasive approaches due to benefits such as reduced tissue disruption, faster recovery, and lower complication rates. This shift is creating strong demand for next-generation fusion systems and motion-preservation implants optimized for precision-guided procedures.
Emerging markets across Asia Pacific, Latin America, and the Middle East present substantial untapped potential as investments in hospitals, specialty spine centers, and surgical training programs increase. Rising awareness of spinal disorders, improved access to diagnostic imaging, and growing medical tourism are further boosting procedure volumes. Technological innovation focused on lightweight materials, enhanced biomechanics, and patient-specific implant designs is improving long-term outcomes and broadening clinical indications. Strategic collaborations between manufacturers, hospitals, and research institutions are accelerating product adoption and surgeon education. As healthcare systems prioritize advanced musculoskeletal care, these factors are expected to unlock sustained long-term growth opportunities.
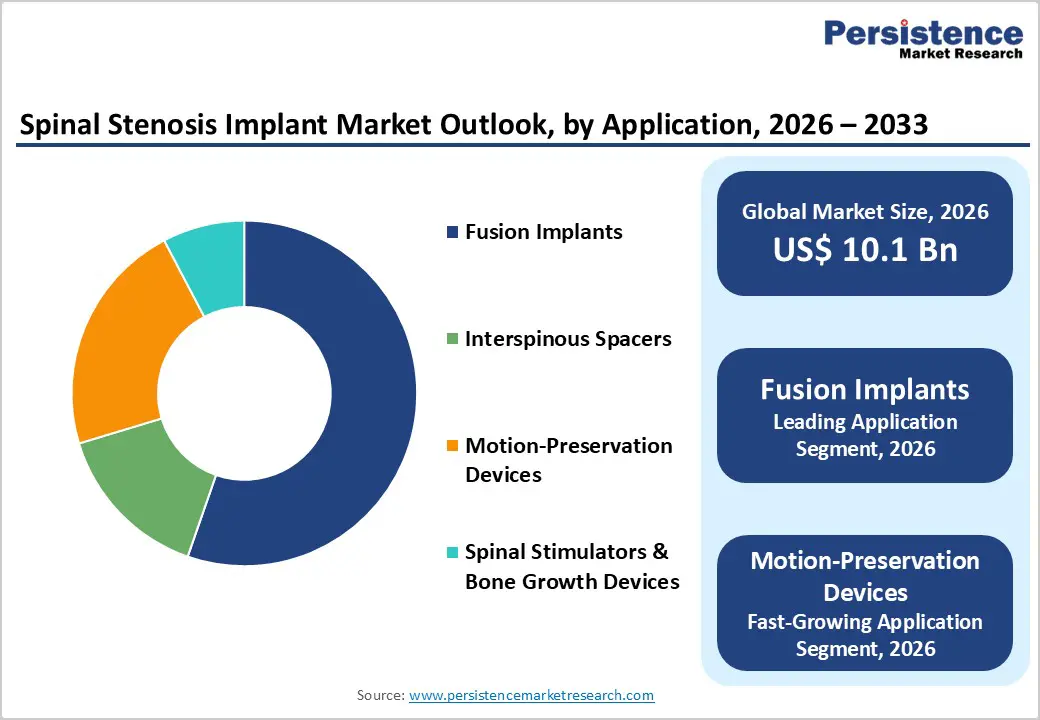
Fusion implants are projected to dominate the global spinal stenosis implant market in 2026, accounting for a revenue share of 55.3%. Their leadership is primarily driven by strong clinical acceptance, proven long-term outcomes, and their effectiveness in stabilizing the spine following decompression procedures. Fusion implants are widely used in moderate to severe cases of spinal stenosis where structural stability and pain relief are critical. Surgeons prefer these implants due to predictable fusion rates, well-established surgical techniques, and broad compatibility with minimally invasive and open surgical approaches. Technological advancements in materials, including titanium and PEEK-based systems, have improved biomechanical performance and reduced complication rates. Additionally, increasing procedure volumes among aging populations and higher adoption in hospitals and specialty spine centers continue to support strong demand. Ongoing innovations focused on reducing surgical time, enhancing fixation strength, and improving patient recovery are expected to reinforce the dominance of fusion implants throughout the forecast period.
The cervical segment is expected to dominate the global spinal stenosis implant market in 2026, capturing a revenue share of 30.0%. This leadership is driven by the high prevalence of cervical spinal stenosis, particularly among aging populations experiencing degenerative disc disease, osteophyte formation, and spinal cord compression. Cervical stenosis often presents with neurological symptoms such as numbness, weakness, and gait instability, prompting earlier diagnosis and surgical intervention compared to other anatomical regions. Implant-based surgical solutions are commonly employed to decompress neural structures while maintaining spinal alignment and mobility. Advances in cervical fusion systems and motion-preservation implants have further expanded treatment options. Growing awareness among clinicians and patients regarding early surgical management to prevent irreversible neurological damage supports consistent procedural volumes. As cervical spine conditions increasingly require timely intervention, this anatomical segment is expected to maintain its leading position in the market.
Hospitals are projected to dominate the global spinal stenosis implant market in 2026, accounting for a revenue share of 62.1%. This dominance is attributed to high patient inflow, availability of experienced spine surgeons, and access to advanced diagnostic imaging and surgical infrastructure. Hospitals routinely manage complex spinal stenosis cases, including multilevel disease, revision surgeries, and patients with comorbidities that require comprehensive perioperative care. Strong adherence to clinical protocols, infection control standards, and post-operative rehabilitation pathways further supports consistent implant utilization. While ambulatory surgical centers and specialty clinics are expanding rapidly, hospitals continue to lead due to their ability to handle high-risk procedures and provide integrated multidisciplinary care. Increasing spinal surgery volumes among elderly populations, rising hospital-based minimally invasive spine procedures, and continued investment in advanced surgical technologies ensure sustained leadership of hospitals within the end-user landscape.
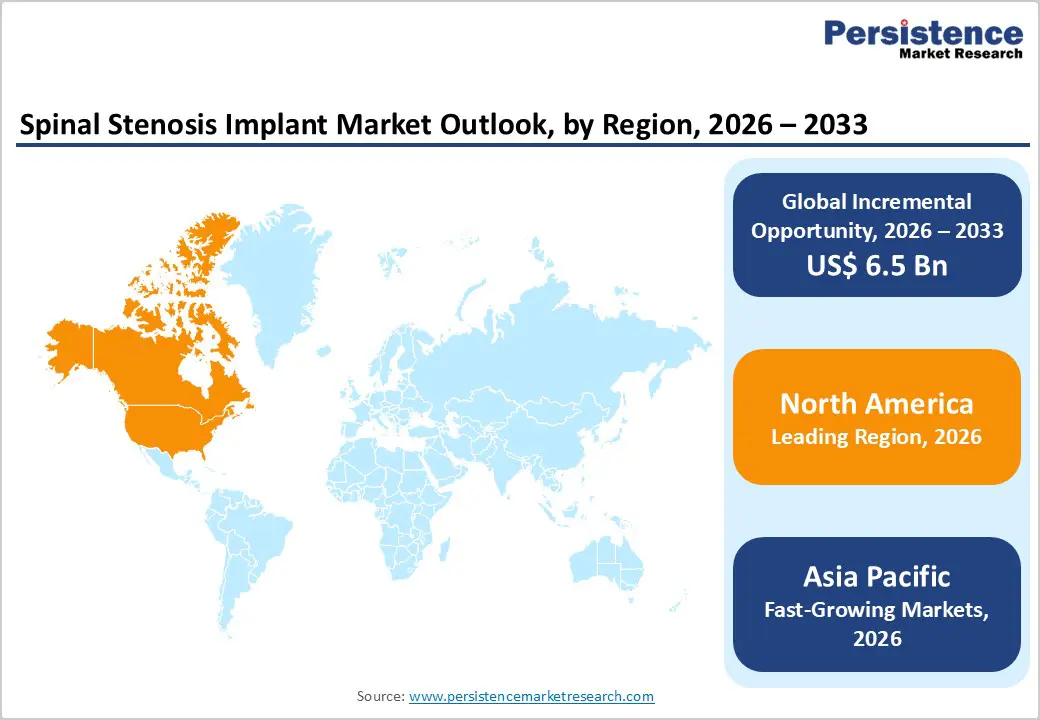
The Europe spinal stenosis implant market is expected to grow steadily, supported by well-established healthcare systems, standardized clinical guidelines, and strong regulatory oversight. Countries such as Germany, the U.K., France, Italy, and Spain account for a significant share of regional demand due to aging demographics and increasing incidence of degenerative spinal conditions. European healthcare providers emphasize early diagnosis and evidence-based treatment, driving consistent utilization of spinal implants. Adoption of minimally invasive surgical techniques is increasing, particularly in tertiary hospitals and specialized spine centers. Strict compliance with medical device regulations encourages the use of high-quality, clinically validated implant systems.
Expansion of outpatient spine services and cross-border healthcare access further supports procedural growth. Additionally, ongoing investments in healthcare infrastructure and surgeon training programs contribute to sustained demand. These factors collectively support stable, long-term growth of the spinal stenosis implant market across Europe.
The Europe spinal stenosis implant market is expected to grow steadily, supported by strong regulatory oversight, standardized clinical guidelines, and widespread access to urology services across countries such as Germany, the U.K., France, Italy, and Spain. The region benefits from an aging demographic profile, which is closely associated with higher prevalence of urethral strictures and lower urinary tract disorders. Public and private healthcare systems emphasize early diagnosis and minimally invasive management, driving consistent demand for urethral dilation procedures.
European healthcare providers place strong emphasis on device safety, material quality, and compliance with medical device regulations, favoring adoption of high-quality disposable and reusable dilators. Expansion of outpatient urology clinics and ambulatory surgical centers is improving procedural accessibility. Additionally, cross-border healthcare and medical tourism in select European countries are contributing to procedural volumes. These factors are expected to support sustained, long-term market growth across the region.
The Asia Pacific spinal stenosis implant market is expected to register a relatively higher CAGR of around 7.2% between 2026 and 2033, driven by improving healthcare infrastructure and rising prevalence of spinal degenerative disorders. Countries such as China, India, Japan, South Korea, and Australia are witnessing increased diagnosis rates due to better access to imaging technologies and growing awareness of spinal health.
Rapid population aging, urbanization, and lifestyle-related musculoskeletal issues are contributing to higher procedural demand. Expansion of private hospitals, specialty spine centers, and ambulatory surgical facilities is improving access to advanced spinal surgeries. The entry of regional manufacturers and availability of cost-effective implant systems are enhancing affordability in price-sensitive markets. Government initiatives aimed at strengthening surgical capacity and growing medical tourism further support market expansion. With improving clinical expertise and acceptance of implant-based spinal care, Asia Pacific is expected to emerge as the fastest-growing regional market.
The global spinal stenosis implant market is highly competitive, with strong participation from companies such as B. Braun SE, BD, Cook, MED pro Medical BV, and Teleflex Inc. Abbott, Stryker, ATEC Spine, Inc, Aurora Spine, Inc, and B. Braun SE. These players leverage extensive global distribution networks, strong brand recognition, and diversified spine-focused product portfolios to address the growing demand for safe, effective, and minimally invasive solutions for spinal stenosis treatment.
Their offerings emphasize implant durability, biomechanical stability, precision engineering, patient safety, surgical ease, and compatibility across multiple clinical applications including lumbar and cervical spinal stenosis management, decompression procedures, and motion-preservation interventions. Continuous technological innovation, regulatory approvals, long-term clinical evidence, compliance with sterilization and biocompatibility standards, and adherence to international quality and manufacturing norms remain critical for sustaining competitive positioning in the global spinal stenosis implant market.
The global spinal stenosis implant market is projected to be valued at US$ 10.1 Bn in 2026.
The global spinal stenosis implant market is driven by the growing aging population and increasing prevalence of degenerative spinal disorders, advancements in minimally invasive surgical techniques and implant technologies, and rising healthcare awareness and infrastructure worldwide.
The global spinal stenosis implant market is poised to witness a CAGR of 5.3% between 2026 and 2033.
Key opportunities include expansion into emerging healthcare markets and the development of advanced minimally invasive and AI-enabled surgical implant solutions.
Abbott, Stryker, ATEC Spine, Inc, Aurora Spine, Inc, and B. Braun SE are some of the key players in the body spinal stenosis implant market.
| Report Attribute | Details |
|---|---|
|
Historical Data/Actuals |
2020 – 2025 |
|
Forecast Period |
2026 – 2033 |
|
Market Analysis |
Value: US$ Bn Volume (in units) If Applicable |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
By Product
By Anatomical Area
By End User
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author