ID: PMRREP35853| 184 Pages | 14 Nov 2025 | Format: PDF, Excel, PPT* | Healthcare

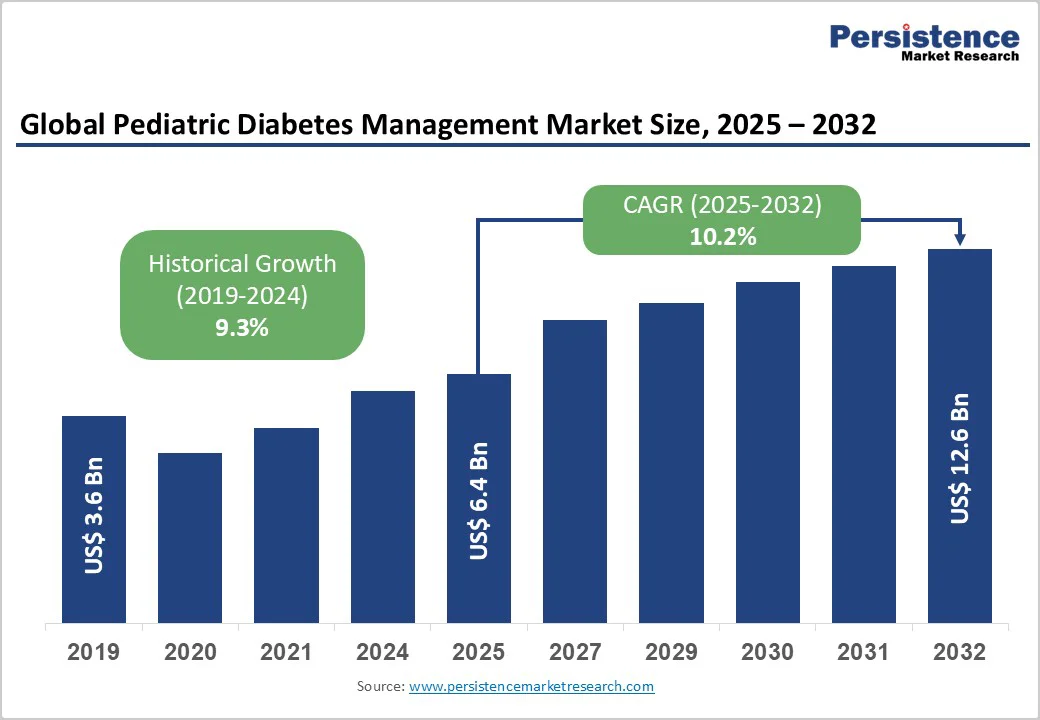
The global pediatric diabetes management market is expected to reach US$6.4 billion in 2025. It is estimated to reach US$12.6 billion in 2032, growing at a CAGR of 10.2% during the forecast period 2025 - 2032, augmented by the increasing incidence of Type 1 and Type 2 diabetes among children as well as the rising demand for technology-enabled care.
| Key Insights | Details |
|---|---|
| Pediatric Diabetes Management Market Size (2025E) | US$6.4 Bn |
| Market Value Forecast (2032F) | US$12.6 Bn |
| Projected Growth (CAGR 2025 to 2032) | 10.2% |
| Historical Market Growth (CAGR 2019 to 2024) | 9.3% |
The steady rise in Type 1 diabetes among children is prompting greater reliance on specialized diabetes management tools. According to the International Diabetes Federation, nearly 1.5 million children and adolescents worldwide are now living with Type 1 diabetes, with annual growth seen across North America, Europe, and Asia. Genetic predisposition and early-life viral infections are contributing to this surge.
Hence, parents and clinicians are increasingly adopting continuous glucose monitors and automated insulin delivery systems to improve safety and quality of life. Pediatric-focused product designs, such as the Dexcom G7’s small sensor and Omnipod 5’s tubeless pump, are direct responses to the needs of this surging population.
Government agencies and healthcare authorities are expanding initiatives to improve diabetes care for children. The U.S. Food and Drug Administration (FDA) has approved new treatment options, such as AstraZeneca’s Farxiga, for pediatric Type 2 diabetes. At the same time, Europe-based regulators have updated device approval pathways to encourage child-specific trials.
Public reimbursement schemes in Germany and Belgium now fully cover CGMs and insulin pumps for children under 18, ensuring broad access to technology-driven management. Organizations such as the WHO and ISPAD are also supporting early screening programs and global awareness campaigns that encourage early diagnosis and the adoption of technologies in pediatric diabetes care.
Managing diabetes in children is uniquely challenging owing to their irregular eating habits and varying levels of physical activity. Children often skip meals, overeat, or engage in spontaneous play, all of which can cause sudden spikes or drops in blood sugar. A 2024 study in the Journal of Pediatric Endocrinology and Metabolism found that inconsistent meal timing and activity patterns were associated with greater glycemic variability in school-aged children than in adults.
Even with novel devices such as CGMs and insulin pumps, maintaining stable glucose levels remains difficult, as these tools rely on predictable patterns to optimize insulin dosing. This unpredictability often demands constant parental supervision and frequent recalibration of treatment plans.
Several schools still lack trained medical staff capable of handling diabetes-related emergencies, leaving children vulnerable during critical moments. The American Diabetes Association reported in 2024 that fewer than 40% of U.S. schools have a full-time nurse, and the numbers are even lower in rural districts.
This gap compels reliance on teachers or administrators, who may not be properly trained in glucose monitoring or insulin administration. Inadequate school preparedness often delays intervention during hypoglycemic or hyperglycemic events, putting children at risk. Parents are increasingly pushing for structured diabetes management policies and staff training programs across educational institutions.
The introduction of Automated Insulin Delivery (AID) systems is revolutionizing diabetes care for children by combining continuous glucose monitoring with smart insulin pumps. These systems automatically adjust insulin doses based on glucose readings, reducing parental stress and minimizing manual intervention.
A 2024 clinical trial published in Diabetes Technology & Therapeutics found that children using hybrid closed-loop systems achieved up to 20% better glycemic control than traditional pump users. As AI systems become more compact, affordable, and AI-based, their adoption in pediatric care is predicted to accelerate globally.
The rising adoption of telemedicine is improving diabetes management for children, especially in regions with limited endocrinology specialists. Remote monitoring platforms now allow healthcare providers to review glucose data from CGMs and pumps in real time and adjust insulin doses without in-person visits. From 2023 to 2024, leading pediatric centers such as Boston Children’s Hospital reported increased engagement through teleconsultations, with better adherence to therapy among young patients.
Mobile apps such as LibreLink and Glooko are also enabling parents to share data instantly with clinicians. This virtual connectivity ensures continuous follow-up, reduces travel burden for families, and supports early intervention in case of glycemic instability, making telemedicine a key growth avenue in pediatric diabetes management.
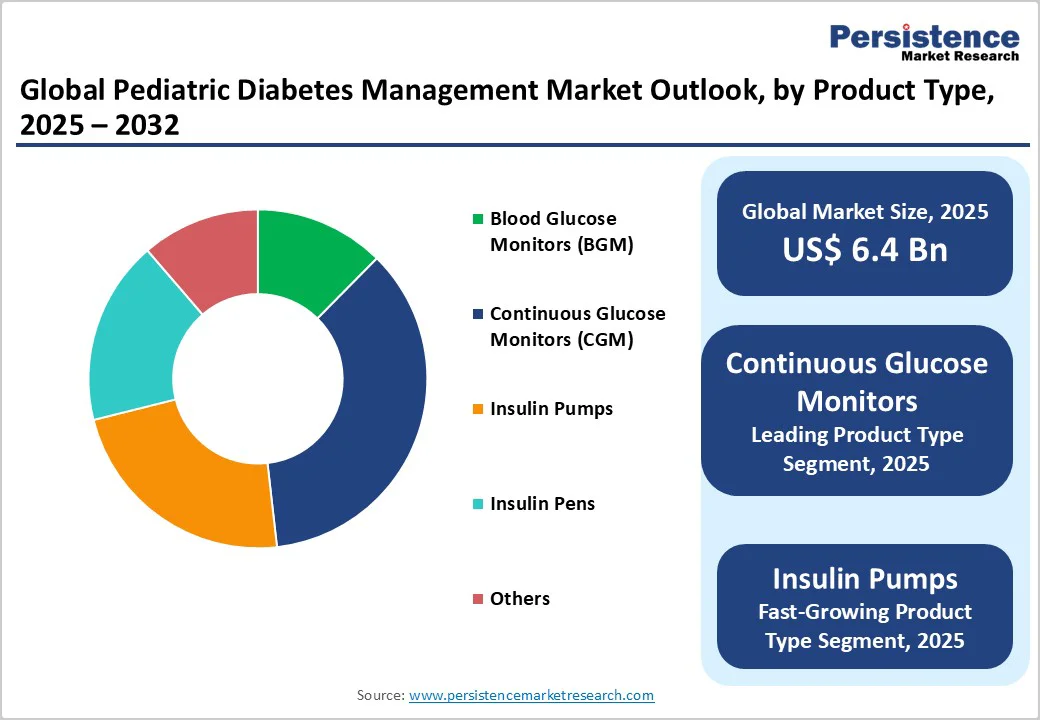
Continuous Glucose Monitors (CGM) are expected to account for nearly 35.8% of the market share in 2025 as they provide real-time and continuous tracking of blood glucose levels, eliminating the requirement for frequent finger pricks.
This technology allows for early detection of hypo- and hyperglycemia, which is important for children who may not always recognize symptoms. Recent studies showed that CGM use in children with Type 1 diabetes improved time-in-range by over 15% compared to traditional glucose monitoring.
Insulin pumps are witnessing steady demand as they provide precise and flexible insulin delivery that mimics natural pancreatic function. For pediatric patients, pumps reduce multiple daily injections and make dose adjustments easier during school hours or physical activity. Many children and parents prefer pumps due to customizable dosing schedules and discreet usage, which support better adherence and quality of life.
Connected devices are predicted to capture approximately 63.2% of the market share in 2025, as they enable remote monitoring and data sharing with caregivers and healthcare professionals. Parents can track glucose trends and insulin doses through smartphones, receiving alerts if glucose levels drop dangerously low.
In 2024, Dexcom and Garmin’s smartwatch integration made glucose tracking more accessible during physical activities, especially for active children. This connectivity supports telehealth consultations and continuous care, catering to modern digital healthcare models.
Non-connected devices are gaining impetus mainly in developing countries, where affordability and simplicity are top priorities. Several families prefer traditional blood glucose meters or non-connected pumps as they have low upfront costs and do not require stable internet access or app compatibility.
Manufacturers are improving these basic models by adding features such as long battery life and simplified displays. In areas with limited digital literacy, non-connected devices still play an essential role in maintaining consistent diabetes management without the complexity of data syncing or subscription-based apps.
Hospitals are speculated to hold around 43.3% of the share in 2025, as they handle initial diabetes diagnosis, device training, and regular follow-ups for pediatric patients. Specialized pediatric endocrinology units in hospitals often serve as the first point of care, helping families choose suitable devices and learn safe insulin dosing. Various hospitals also run CGM initiation programs and conduct controlled trials for new devices before they are prescribed for home use.
Homecare is emerging as a key end-user due to the surging emphasis on self-management and remote monitoring. Families prefer managing diabetes at home to minimize hospital visits, especially for children. Devices such as the Omnipod 5 tubeless pump and LibreLink app allow parents to monitor glucose data from home, even when the child is at school. With rising telemedicine adoption, doctors can adjust treatment plans remotely based on home-recorded data.
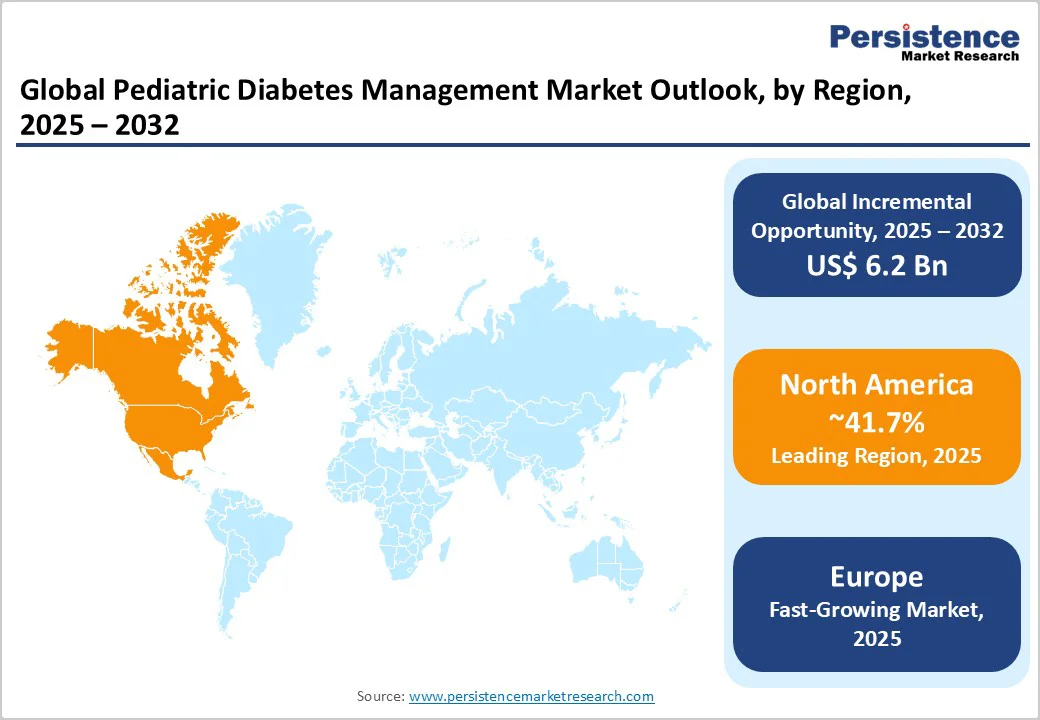
North America is estimated to account for approximately 41.7% of share in 2025, owing to the widespread use of Continuous Glucose Monitors (CGMs) and insulin pumps. The U.S. has seen a surging preference for smart diabetes technologies such as Dexcom G7, Medtronic MiniMed 780G, and Tandem t:slim X2, which automate insulin delivery and reduce parental monitoring stress.
The FDA’s recent approval of AstraZeneca’s Farxiga for children aged 10 and above has also expanded treatment options for Type 2 diabetes in kids. Disparities persist; rural areas and low-income families often face limited access due to high device costs and insurance variability. In addition, the rising incidence of Type 2 diabetes among adolescents, pushed by obesity and lifestyle factors, has made early detection and digital health programs a regional priority.
In Europe, the pediatric diabetes management market is characterized by superior public healthcare support and high adoption of technology. Germany, Sweden, and Denmark have some of the highest rates of insulin pump and CGM use among children with Type 1 diabetes, supported by national reimbursement policies. Belgium recently expanded full coverage for continuous and flash glucose monitoring for all children under 18, signaling increasing policy alignment toward universal access.
Differences persist across countries. While Nordic and Western European nations provide comprehensive coverage, Eastern European regions still struggle with affordability and limited device penetration. The EU’s pediatric regulation framework has encouraged more clinical trials and product labeling for children, which continues to influence development across the region.
In Asia Pacific, pediatric diabetes management is surging considerably but remains uneven across countries. China and India are witnessing a surge in childhood diabetes cases, prompting hospitals and private players to introduce mobile health apps and CGM-based care programs in major cities. Abbott and Medtronic have expanded their presence in urban areas, but high costs and limited reimbursement restrict rural access.
Governments in Japan and Australia are promoting early screening and digital diabetes education programs in schools, improving diagnosis rates. Despite this, most Asia Pacific markets still rely on out-of-pocket spending, and shortages of pediatric endocrinologists remain a challenge. However, affordable CGMs and smartphone-integrated glucose monitoring systems are anticipated to bridge accessibility gaps over the next ten years.
The global pediatric diabetes management market is highly competitive, with leading players such as Medtronic, Dexcom, Abbott, Insulet, Tandem Diabetes Care, Novo Nordisk, Eli Lilly, and Sanofi dominating the landscape. Device makers focus on CGM systems and insulin pumps that are specifically designed for children.
Pharmaceutical companies are innovating in insulin formulations and digital support programs. The launch of Dexcom G7 and Abbott’s FreeStyle Libre 3 systems has intensified competition, as both devices deliver small sensors and improved accuracy suitable for pediatric use.
The pediatric diabetes management market is projected to reach US$6.4 Billion in 2025.
Rising incidence of Type 1 diabetes among children and supportive regulatory approvals are the key market drivers.
The pediatric diabetes management market is poised to witness a CAGR of 10.2% from 2025 to 2032.
The development of hybrid closed-loop systems and the expansion of telehealth are the key market opportunities.
DexCom, Inc., Tandem Diabetes Care, Inc., and Abbott Laboratories are a few key market players.
| Report Attribute | Details |
|---|---|
| Historical Data/Actuals | 2019 - 2024 |
| Forecast Period | 2025 - 2032 |
| Market Analysis | Value: US$ Bn |
| Geographical Coverage |
|
| Segmental Coverage |
|
| Competitive Analysis |
|
| Report Highlights |
|
By Product Type
By Technology
By End-user
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author