ID: PMRREP35328| 199 Pages | 16 May 2025 | Format: PDF, Excel, PPT* | Healthcare

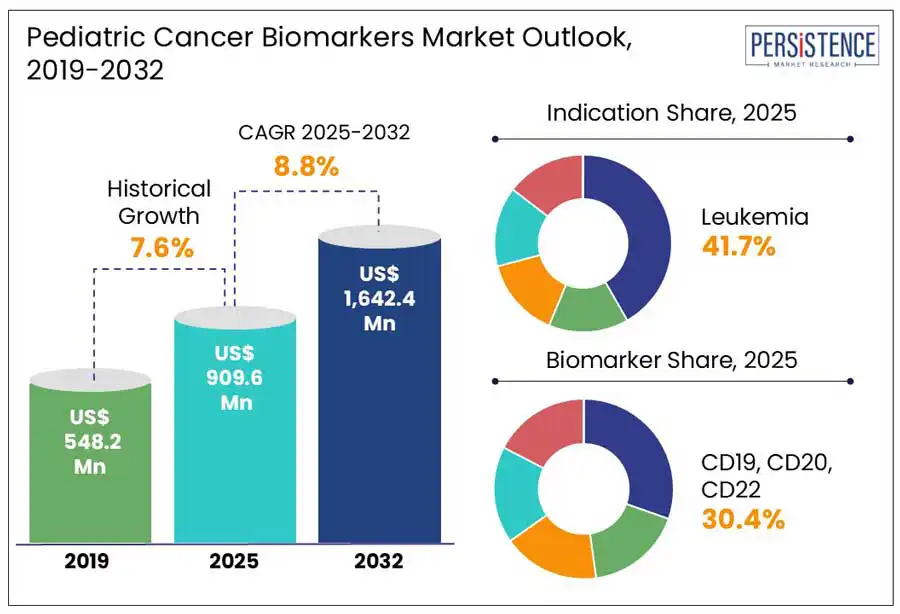
The global pediatric cancer biomarkers market size is predicted to reach US$ 1,642.4 Mn in 2032 from US$ 909.6 Mn in 2025. It will likely witness a CAGR of around 8.8% in the forecast period between 2025 and 2032.
Pediatric cancer biomarkers have become significant for the management, treatment, and diagnosis of childhood cancers. With childhood cancers being biologically distinct from adult cancers, the role of biomarkers has become important in coming up with personalized treatment strategies. Their development is estimated to be propelled by technological innovations in metabolomics, proteomics, and genomics.
Key Industry Highlights
|
Global Market Attribute |
Key Insights |
|
Pediatric Cancer Biomarkers Market Size (2025E) |
US$ 909.6 Mn |
|
Market Value Forecast (2032F) |
US$ 1,642.4 Mn |
|
Projected Growth (CAGR 2025 to 2032) |
8.8% |
|
Historical Market Growth (CAGR 2019 to 2024) |
7.6% |
Surging emphasis on early diagnosis and screening is poised to augment the pediatric cancer biomarkers market growth through 2032, states Persistence Market Research. With about 400,000 children and adolescents aged 0 to 19 diagnosed with cancer worldwide each year, early detection has become a significant focus in pediatric oncology, stated the World Health Organization (WHO). This shift is propelled by the notion that early detection of cancer leads to more effective and less aggressive treatments, enhancing quality of life and survival rates for young patients. Demand for biomarker-based diagnostic tests capable of detecting cancer at its early stage is hence envisioned to rise.
The pediatric Acute Myeloid Leukemia (AML) biomarker testing market is also witnessing steady growth as biomarker testing plays an important role in early diagnosis and treatment planning. A 2024 BMC Cancer study using Artificial Intelligence (AI) scrutinized blood samples from children with AML and detected significant variations in biochemical indicators compared to healthy children. The study further utilized a random forest model, obtaining an area under the curve (AUC) of 0.909 for predicting AML, showing the potential of machine learning in improving early detection accuracy. These findings highlight the significance of integrating innovative technologies and biomarker analysis in the early diagnosis of AML, spurring targeted and timely therapeutic interventions.
The genetic complexity of pediatric cancer is predicted to hinder demand for specific biomarkers in the forecast period. Genetic heterogeneity in pediatric tumors adds a layer of complexity. Unlike adult cancers, which tend to have well-characterized mutations, pediatric tumors often showcase less understood and diverse genetic alterations. This makes it challenging to detect universal biomarkers applicable across multiple cases. The variability requires more personalized approaches, which are both time-consuming and resource-intensive. Hence, genetic diversity in pediatric cancers will likely hamper the development and adoption of standardized biomarker-based diagnostics.
The metabolomics biomarker market, however, is poised to create promising opportunities in the field of pediatric cancer. Researchers are anticipated to discover specific biomarkers that reflect the tumor's biochemical state by scrutinizing the unique metabolic profiles of cancer cells. A study published in PLOS ONE in June 2023 found distinct metabolic patterns in pediatric medulloblastoma patients compared to healthy controls. It used ultra-performance liquid chromatography along with mass spectrometry (UPLC-Q/E-MS/MS). The study detected 25 significantly altered metabolites, with six exhibiting high specificity and precision as diagnostic biomarkers. Similar studies suggest that metabolomics is capable of offering valuable insights into tumor biology and helps in the early detection of pediatric cancer.
Personalized medicine is anticipated to create lucrative avenues in the field of pediatric cancer biomarkers. These enable the development of treatments that cater to the unique genetic profiles of specific tumors. A study conducted at Sydney Children's Hospital recently found that around 55% of children receiving personalized therapies achieved complete or partial remission, compared to only 12% in the standard treatment group. This approach not only enhances tumor response rates but also improves long-term survival prospects. It is evident in patients with aggressive cancers previously considered difficult to treat.
The next-generation biomarker market is also predicted to play a key role in the foreseeable future. The integration of Next-Generation Sequencing (NGS) technologies, including RNA sequencing and Whole-Exome Sequencing (WES), into pediatric oncology has extended the utility of biomarkers. NGS has offered clinically impactful findings in nearly 66% of pediatric hematologic malignancy cases in clinical settings. These technologies have helped uncover pharmacogenomic modifiers and detect actionable mutations.
Based on indication, the market is divided into leukemia, neuroblastoma, CNS tumors, and lymphoma. Out of these, the leukemia segment is anticipated to hold a share of approximately 41.7% in 2025. It has become a key focus of various healthcare institutions due to its well-characterized genetic alterations and high prevalence rate. As per the Leukemia and Lymphoma Society, leukemia accounts for around 25.4% of all childhood cancer cases in the U.S. alone. Among these, Acute Lymphoblastic Leukemia (ALL) comprises nearly 75 to 80%, making it the most prominent form of leukemia in children.
The high prevalence offers a significant patient population for clinical trials and research, bolstering the validation and identification of biomarkers. With innovative genomic technologies and biomarker-driven approaches, the leukemia screening market is predicted to continue its expansion in the forecast period.
The neuroblastoma market, on the other hand, is envisioned to exhibit a considerable CAGR from 2025 to 2032. As per a study published in the National Library of Medicine, neuroblastoma accounts for approximately 8 to 10% of all childhood cancers worldwide, making it a common extracranial solid tumor in pediatric populations. There were nearly 1,977 deaths and 5,560 new cases attributed to neuroblastoma in 2021, pointing to its significant impact on children’s health. The disease is characterized by its molecular heterogeneity with important biomarkers such as ALK mutations and MYCN amplification. The identification of these biomarkers is considered significant for the development of targeted therapies.
In terms of biomarkers, the market is segregated into alpha-fetoprotein, neuron-specific enolase, CD19, CD20, CD22, and ALK. Among these, CD19, CD20, and CD22 are projected to account for nearly 30.4% of the pediatric cancer biomarkers market share in 2025. These are considered key biomarkers in pediatric cancer, mainly in B-cell Precursor Acute Lymphoblastic Leukemia (BCP-ALL). It is due to their high therapeutic targeting potential, roles in B-cell development, and consistent expression patterns. CD19 is perceived to be a cornerstone in diagnosis and minimal residual disease monitoring. CD20 is expressed in about 40 to 50% of BCP-ALL cases, with its expression levels varying among patients. CD22 is consistently expressed on BCP-ALL blasts and plays an important role in B-cell receptor signaling.
Anaplastic Lymphoma Kinase (ALK) will likely showcase a steady growth rate in the diagnostic biomarker market through 2032. In neuroblastoma, ALK mutations are present in about 8 to 10% of primary tumors, with the most common mutations occurring at positions R1275, F1174, and F1245 within the tyrosine kinase domain. These mutations lead to constitutive activation of ALK, promoting tumor cell proliferation and survival. ALK inhibitors such as crizotinib have exhibited potential in treating ALK-positive neuroblastoma. Crizotinib has demonstrated efficacy in preclinical models and early-phase clinical trials, resulting in its investigation in pediatric neuroblastoma patients.
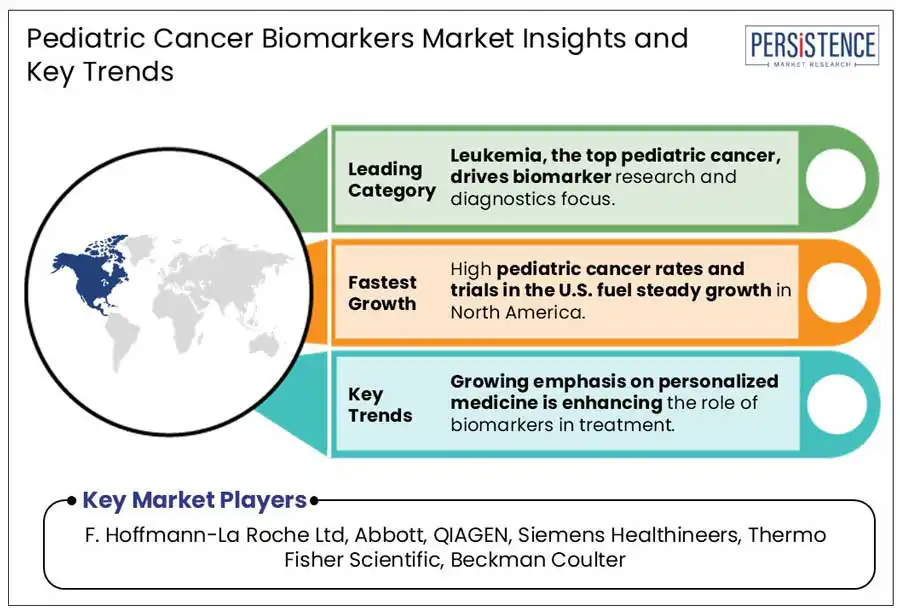
In 2025, North America is expected to generate a share of about 44.2%. The U.S. pediatric cancer biomarkers market is projected to remain at the forefront of growth due to significant federal investments in research and development activities. The Childhood Cancer Data Initiative (CCDI), which was rolled out in 2019 with an annual budget of US$ 50 Mn over 10 years, focuses on boosting data sharing and research in pediatric oncology. In addition, advocacy groups such as the American Cancer Society Cancer Action Network (ACS CAN) have taken initiatives to raise more funds, including a US$ 30 Mn allocation for the STAR Act to support research, treatment access, and survivorship.
The personalized medicine biomarkers market is also transforming pediatric cancer care in the U.S. by developing treatments catering to the molecular and genetic profiles of individual tumors. Legislative measures are expected to support the integration of biomarker testing into pediatric cancer care. Kentucky, for example, passed House Bill 180 in 2023, mandating health insurers to cover biomarker tests for cancer patients. It hence facilitated more customized and effective treatments. Similarly, Connecticut's HB 6771 focuses on offering insurance coverage for biomarker testing, emphasizing its potential to develop treatments.
In Europe, the medical industry is characterized by the collaborative efforts among regulatory bodies, healthcare providers, and research institutions. Leukemia posed as one of the most prominent and preferred indications in France in 2024, while cases of Central Nervous System (CNS) tumors showed significant growth. Several research institutions are striving to discover novel pediatric cancer biomarkers. The Glasgow Precision Oncology Laboratory (GPOL) at the University of Glasgow has been joining hands with the National Health Service (NHS) and other industry partners to research the development of novel therapeutic strategies.
The ONCOCHECK project in Spain is supported by the European Union's Horizon 2020 research and innovation program. It aims to clinically validate Telomere-Associated Variables (TAVs) as cancer biomarkers. The project involves more than 1,500 participants, including children with several types of cancer. The European Society for Pediatric Oncology (SIOPE) plays a key role in advocating for integrating biomarker testing in pediatric cancer care. It has been involved in various European Union-funded projects such as the European Reference Network on Pediatric Cancer (ERN PaedCan) and the Joint Action on Rare Cancers (JARC), thereby spurring developments in the cancer diagnostics market.
In Asia Pacific, India and China are currently seeing a significant surge in pediatric cancer cases. These are further compelling healthcare organizations and governments to invest in cancer research and treatment facilities. China, for example, reports more than 4.5 Mn new cancer cases every year, making early diagnosis important. Biomarkers allow targeted therapies and precise diagnostics, reducing healthcare costs and improving patient outcomes. In addition, the renal biomarker market has gained traction as the prevalence of kidney-related conditions rises among pediatric cancer patients.
Technological innovations are further expected to drive the market in Asia Pacific with surging emphasis on genomics and precision medicine. NGS technologies have transformed biomarker discovery, enabling the identification of molecular signatures for several diseases. As per Japan’s Ministry of Health, Labor, and Welfare, genomic medicine programs emphasizing cancer diagnostics have supported biomarker adoption. India’s Genome India Project also focuses on mapping the genetic makeup of its population, improving the understanding of disease pathways.
The global pediatric cancer biomarkers market is characterized by a dynamic landscape with various key companies and niche players boosting growth. Leading companies are leveraging their extensive genomic and proteomic platforms to enhance biomarker discovery. Illumina, for instance, has already made strides with its FDA-approved TruSight Oncology test, made for use in both adult and pediatric cancer patients. The market is also experiencing increasing partnerships between pediatric research institutions and diagnostics firms. These partnerships enable fast regulatory approvals and bolster biomarker discovery.
The market is projected to reach US$ 909.6 Mn in 2025.
Increasing emphasis on early diagnosis and rising government investments in biomarker development programs are the key market drivers.
The market is poised to witness a CAGR of 8.8% from 2025 to 2032.
Surging innovations in metabolomics biomarkers and increasing focus on personalized medicine are the key market opportunities.
F. Hoffmann-La Roche Ltd, Abbott, and QIAGEN are a few key market players.
|
Report Attribute |
Details |
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis Units |
Value: US$ Bn/Mn, Volume: As Applicable |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
|
Customization and Pricing |
Available upon request |
By Indication
By Biomarker
By End-use
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author