ID: PMRREP25708| 234 Pages | 8 Feb 2026 | Format: PDF, Excel, PPT* | Healthcare

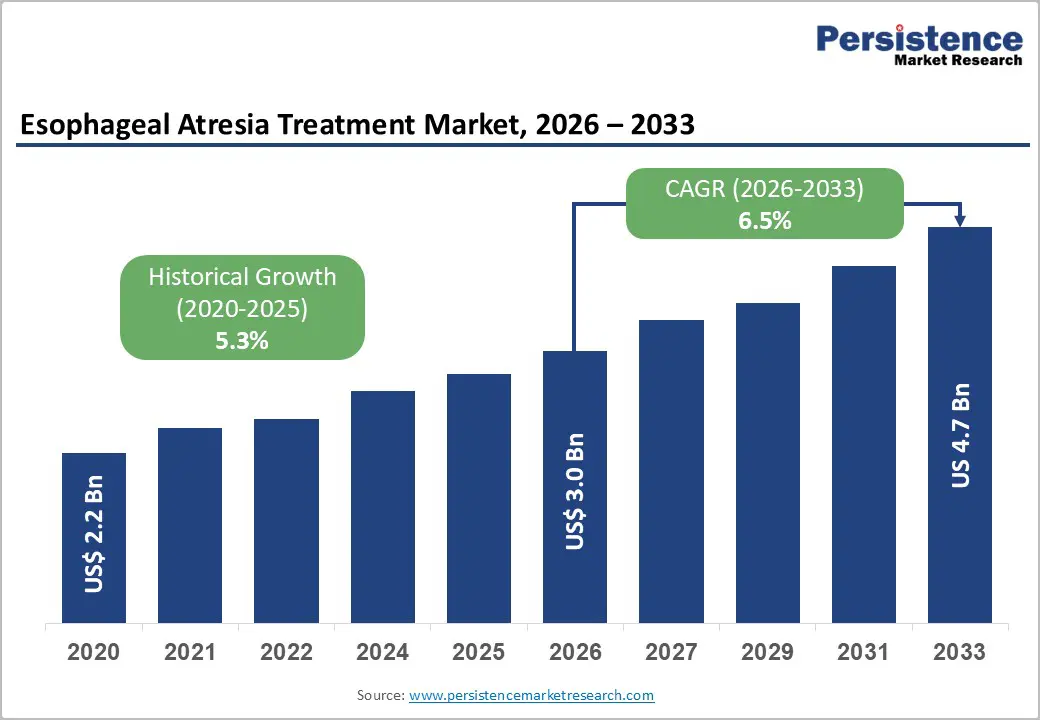
The global esophageal atresia treatment market size is projected to be valued at US$3.0 billion in 2026 and is projected to reach US$4.7 billion by 2033, growing at a CAGR of 6.5% during the forecast period from 2026 to 2033, driven by the rising global incidence of congenital anomalies and the rapid modernization of Neonatal Intensive Care Units (NICUs) across emerging economies. Technological convergence, particularly the integration of robotic-assisted surgical platforms and advanced tissue-engineered scaffolds, is addressing previously unmet needs in Long-Gap Esophageal Atresia (LGEA) management.
| Key Insights | Details |
|---|---|
| Esophageal Atresia Treatment Market Size (2026E) | US$3.0 Bn |
| Market Value Forecast (2033F) | US$4.7 Bn |
| Projected Growth (CAGR 2026 to 2033) | 6.5% |
| Historical Market Growth (CAGR 2020 to 2025) | 5.3% |
Advancements in minimally invasive surgery and robotic integration are expected to remain a central market driver, reshaping procedural preferences across esophageal atresia treatment pathways. The shift from open thoracotomy toward thoracoscopic repair is driven by measurable clinical advantages, including reduced postoperative pain, shorter hospitalization cycles, and lower incidence of long-term musculoskeletal complications. These outcome improvements are reinforcing clinician confidence and accelerating adoption, particularly for the most prevalent esophageal atresia presentations. As healthcare systems increasingly prioritize value-based pediatric care, minimally invasive approaches are projected to sustain procedural demand and elevate overall treatment value without altering established care algorithms.
Robotic-assisted platforms are further strengthening this driver by enhancing surgical precision within the anatomical constraints of neonatal thoracic cavities. Three-dimensional visualization and articulated instrumentation improve anastomotic accuracy and procedural consistency, supporting broader uptake in high-volume pediatric centers. While adoption remains capital-intensive, concentration within advanced surgical hubs is enabling rapid skill standardization and outcome optimization. Over the forecast period, continued integration of robotic technologies is likely to reinforce clinical differentiation, support higher procedural complexity thresholds, and contribute to sustained market expansion through improved long-term patient outcomes.
The requirement for highly specialized surgical expertise is expected to remain a structural market restraint, directly limiting scalable adoption of esophageal atresia repair across regions. Procedural frequency remains extremely low at the individual surgeon level, with most pediatric surgeons performing fewer than two repairs annually, constraining skill accumulation and technique refinement. Epidemiological distribution further exacerbates this limitation, as annual case volumes are fragmented across a large number of pediatric surgery units, resulting in consistently subscale exposure per center. This structural dispersion suppresses procedural confidence and reinforces conservative referral patterns, dampening overall treatment penetration despite stable clinical incidence.
A persistent tension between clinical centralization and geographic accessibility continues to cap effective market expansion. While concentration of care within high-volume reference centers is associated with superior outcomes and protocol standardization, it remains logistically inaccessible for a significant proportion of patients. Conversely, decentralized delivery through local programs often operates below critical expertise thresholds, introducing outcome variability and limiting payer and provider willingness to expand services. As a result, addressable patient pools remain constrained in developed markets, while emerging regions face elevated barriers to program establishment, collectively restricting sustainable market growth.
Integration of regenerative medicine and tissue engineering is projected to represent a high-impact market opportunity, particularly for long-gap esophageal atresia, where conventional surgical options carry lifelong morbidity burdens. Bio-artificial esophageal scaffolds based on decellularized, organ-specific matrices seeded with patient-derived mesenchymal stem cells are expected to address unmet clinical needs by enabling native tissue regeneration rather than anatomical substitution. This approach directly targets limitations associated with gastric pull-ups and colonic interpositions, positioning regenerative grafts as a premium, outcome-driven solution. Commercialization of regenerative esophageal constructs is likely to unlock a niche but high-value opportunity, with market potential estimated at approximately US$ 450 million by 2030, supported by growing translational research momentum.
In October 2024, Harvard Apparatus Regenerative Technology (HART/Biostage) entered into a strategic collaboration with Beijing’s Capital Institute of Pediatrics to accelerate clinical validation of bio-artificial esophageal scaffolds for pediatric atresia. This partnership provides access to the high patient volumes in Asia necessary to expedite the clinical data collection for decellularized, stem-cell-seeded scaffolds, effectively bridging the translational gap to achieve the US$450 million market potential by providing a definitive solution for Long-Gap Esophageal Atresia (LGEA).
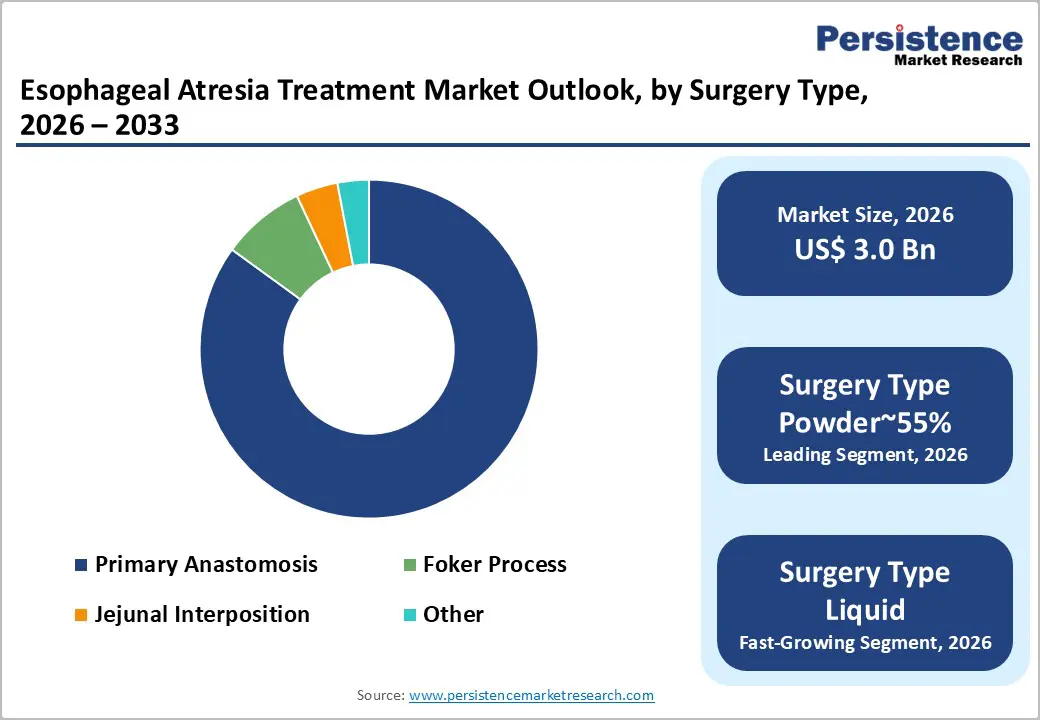
Primary anastomosis is expected to lead the esophageal atresia treatment market, accounting for approximately 85% share in 2026, driven by its role as the definitive single-stage repair for the dominant short-gap Type C anatomy. Surgeons prioritize native-tissue preservation because it delivers superior swallowing outcomes and lower long-term morbidity than gastric or colonic replacements, aligning with hospital cost-containment objectives.
Adoption of thoracoscopic primary repair continues to expand, supported by fluorescence-guided perfusion assessment with indocyanine green to mitigate leak risk and intraoperative gap-measurement tools that optimize tension management. Barbed sutures such as Ethicon V-Lok improve suture-line uniformity in constrained neonatal fields, while ERAS pathways shorten ventilation and NICU utilization. Visualization and ventilation platforms from Medtronic and post-repair dilation pathways using Cook Medical devices reinforce standardized, high-throughput care models in high-volume centers.
The Foker process is expected to be the fastest-growing segment, as referral patterns consolidate complex long-gap cases into specialist centers, prioritizing esophageal preservation. Clinical advocacy for native-tissue continuity accelerates uptake versus replacement procedures, supported by evolution toward minimally invasive internal traction that reduces prolonged immobilization and sedation burden.
Adjuncts such as botulinum toxin–assisted muscle relaxation shorten traction duration, improving tolerance and throughput in neonatal ICUs. High-volume programs refine left-sided MIS approaches to enhance precision, while daily radiographic monitoring and contrast surveillance de-risk staged growth protocols. Device ecosystems from Ethicon and Cook Medical enable reliable traction control, and multidisciplinary pathways integrate airway, nutrition, and reflux management, strengthening outcomes and institutional confidence in growth-induction strategies.
Hospital pharmacies are anticipated to lead the esophageal atresia market, accounting for approximately 70% share in 2026, underpinned by their control over time-critical neonatal medication access and the tightly coupled perioperative supply chain. EA diagnosis at birth concentrates demand within Level III and IV NICUs, where point-of-care compounding systems enable weight-based dosing of antibiotics, surfactants, and reflux therapies within minutes.
Smart cabinet ecosystems from Omnicell and BD Pyxis compress fulfillment latency for high turnover consumables, while pharmacy-led meds-to-beds programs capture discharge-linked device kits and clinical nutrition. Total parenteral nutrition production, contrast media stewardship for esophagrams, and access to rare-size stents create a captive institutional channel. Centralized pediatric formularies across large health systems standardize care bundles, reinforced by RFID inventory control, IV robotic compounding from B. Braun, and wholesaler networks led by Cencora, Baxter, and B. Braun.
Online distribution is expected to be the fastest-growing segment in the esophageal atresia market, propelled by the migration of post-surgical maintenance and chronic care into home settings. Direct-to-patient logistics enable delivery of enteral pumps, dilation kits, and nutrition through encrypted portals, while subscription models stabilize refill adherence for long duration regimens. EHR-integrated ordering automates home-care fulfillment at discharge, reducing gaps in therapy and administrative friction for clinics.
Tele-pharmacy support extends pediatric gastroenterology expertise to caregivers, and AI-driven sizing forecasts anticipate catheter and tube upgrades as infants grow. B2B e-commerce accelerates restocking for community clinics. Trust expands through digital labeling and reimbursable e-pharmacy workflows, with cold-chain visibility and track-and-trace improving reliability. McKesson, Cardinal Health atHome Solutions, and Byram Healthcare anchor scale across consumer and clinic channels.
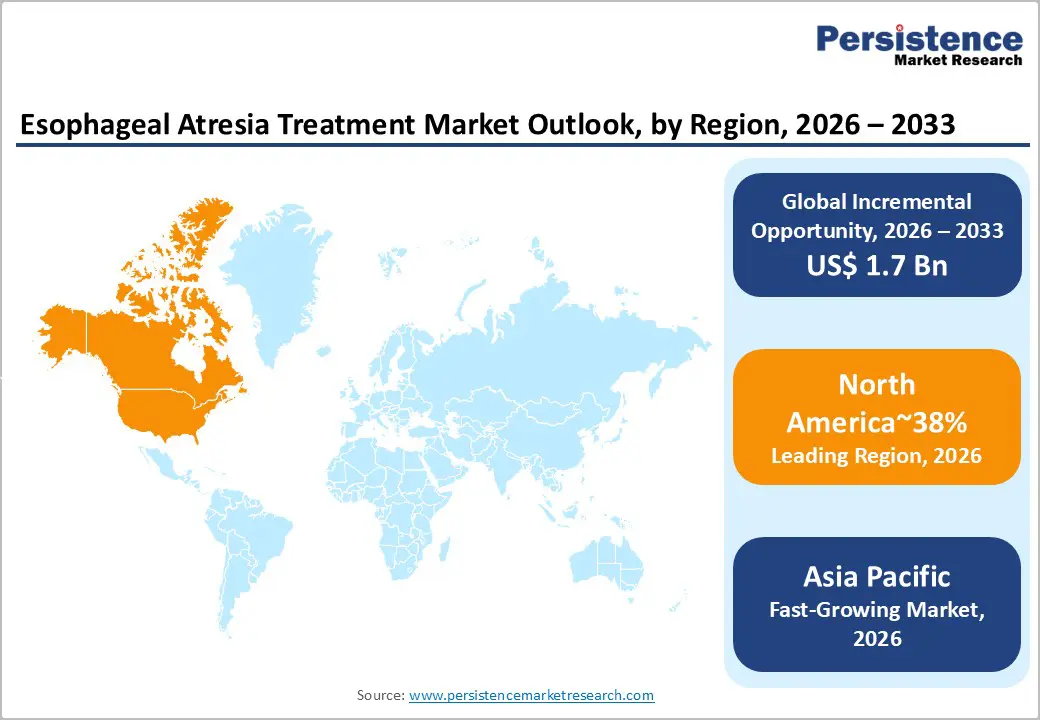
North America is projected to maintain the largest share, accounting for approximately 38% in 2026, supported by robust reimbursement structures and a high concentration of pediatric surgical capacity. The region benefits from advanced neonatal intensive care infrastructure, well-established referral networks, and standardized treatment protocols that facilitate timely intervention for complex cases. Early adoption of robotic-assisted thoracoscopic platforms and the presence of major medical technology headquarters reinforce procedural innovation and optimize clinical outcomes. Regulatory clarity under the FDA’s Humanitarian Device Exemption (HDE) further supports market stability by enabling pediatric-specific device approvals within structured pathways.
Market expansion in North America is moderated by demographic stability and mature adoption of established surgical techniques, resulting in growth slightly below the global average. Strategic opportunities are concentrated in specialty care, including long-gap EA management, post-operative complication protocols, and adult transition programs for patients with prior EA repair. The integrated healthcare ecosystem, combining high procedural capacity, reimbursement support, and centralized expertise, positions North America as the benchmark for clinical standards and market structuring within the global esophageal atresia landscape.
Growth in Europe is underpinned by regulatory harmonization and centralized healthcare systems that support standardized outcome tracking. Key markets, including Germany, the U.K., and France, lead in infrastructure and procedural capacity, with multicenter registries enabling consistent clinical protocols and best-practice dissemination. European Reference Networks, particularly ERNICA, further facilitate concentration of expertise in high-volume centers, enhancing treatment quality and procedural efficiency. Regulatory oversight under the EU Medical Device Regulation reinforces clinical transparency and patient safety, supporting structured adoption of pediatric-specific devices while imposing constraints on smaller manufacturers.
Market expansion is shaped by a balance between centralization and access, as geographic concentration of EA care limits the number of operational centers but enables higher procedural volumes per site, justifying investment in specialized instrumentation and surgeon training. Eastern European countries are emerging as growth nodes, establishing dedicated pediatric surgical capacity to address previously unmet demand. Europe’s combination of policy-driven standardization, robust registry infrastructure, and strategic centralization positions the region for stable, incremental growth within the global market, projected at 5-6% annually.
Asia Pacific is projected to be the fastest-growing region, driven by expanding neonatal healthcare infrastructure, rising middle-class access to private pediatric surgical care, and the consolidation of medical tourism for specialized procedures. Key markets, including China, India, and Japan are leading regional development, with India establishing high-volume pediatric surgical centers that offer advanced NICU capabilities, specialized pediatric surgical teams, and cost-effective treatment pathways. China is expanding domestic pediatric surgical capacity through targeted infrastructure investment and the scaling of specialized training programs, reducing reliance on international referrals. Japan and South Korea contribute with mature healthcare systems and established surgical expertise, combining advanced infrastructure with strong procedural capabilities. The region benefits from operational innovation, where surgical protocols are adapted for cost efficiency without compromising clinical outcomes, supporting wider adoption across urban and semi-urban centers and reinforcing procedural standardization.
Asia Pacific is supported by rising birth volumes, improving diagnostic capabilities, and enhanced access to specialized pediatric care. Growth is further enabled by telemedicine-enabled consultations, structured specialist training programs, and frugal innovation in surgical techniques that optimize outcomes within resource-limited environments. While access disparities persist in rural areas due to limited infrastructure and specialist availability, the ongoing expansion of neonatal facilities and workforce development is progressively bridging these gaps. Collectively, these structural, demographic, and policy factors position Asia Pacific as a high-velocity growth market within the global esophageal atresia landscape, combining scale, efficiency, and improving clinical standards to support sustained adoption and procedural advancement.
The global esophageal atresia treatment market is moderately consolidated, with the top five players controlling approximately 45% of the total revenue, led by large medical device conglomerates that maintain diversified portfolios across surgical instruments, NICU equipment, and haemostasis devices. Competitive positioning is primarily driven by technological expertise, intellectual property protection, and the ability to provide integrated solutions across perioperative and neonatal care pathways. High barriers to entry, including regulatory approvals, specialized R&D requirements, and limited patient populations, reinforce the influence of established manufacturers while constraining scale for new entrants.
Fragmentation persists in specialized traction and regenerative segments, where numerous startups and academic spin-offs drive innovation in pediatric-specific devices and consumables. Moderate consolidation is occurring as major players acquire niche pediatric surgical companies to broaden portfolio coverage and address clinical gaps. Forward-looking trends indicate sustained focus on product innovation, IP-backed differentiation, and integrated care solutions, with market growth shaped by selective expansion, regulatory alignment, and incremental adoption of advanced surgical instrumentation in high-demand geographies.
Key Industry Developments:
The global braiding machine market is projected to be valued at US$0.5 billion in 2026 and is expected to reach US$0.7 billion by 2033, driven by the transition toward high-performance technical braiding for automotive and medical applications.
Demand is shifting due to the rapid expansion of electric vehicle production, which requires lightweight composite materials, and the growing need for high-precision braided components in medical devices, aerospace, and industrial sectors where abrasion resistance and flexibility are critical.
The braiding machine market is forecast to grow at a CAGR of 4.0% from 2026 to 2033, supported by advancements in automation and the rising adoption of smart textiles and composites.
Asia Pacific is the fastest-growing regional market, fueled by rapid industrial modernization, massive infrastructure projects in China and India, government-backed innovation programs, and the widespread adoption of IoT-enabled manufacturing.
Key players include Herzog GmbH, Mayer & Cie., OMA, Steeger, and Wardwell, among others. These companies dominate the high-end segment, focusing on precision engineering and automation for advanced technical applications.
| Report Attribute | Details |
|---|---|
| Historical Data/Actuals | 2020 – 2025 |
| Forecast Period | 2026 – 2033 |
| Market Analysis | Value: US$ Bn |
| Geographical Coverage |
|
| Segmental Coverage |
|
| Competitive Analysis |
|
| Report Highlights |
|
By Surgery Type
By Treatment Approach
By Distribution Channel
By End-user
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author