ID: PMRREP35457| 191 Pages | 3 Jul 2025 | Format: PDF, Excel, PPT* | Healthcare

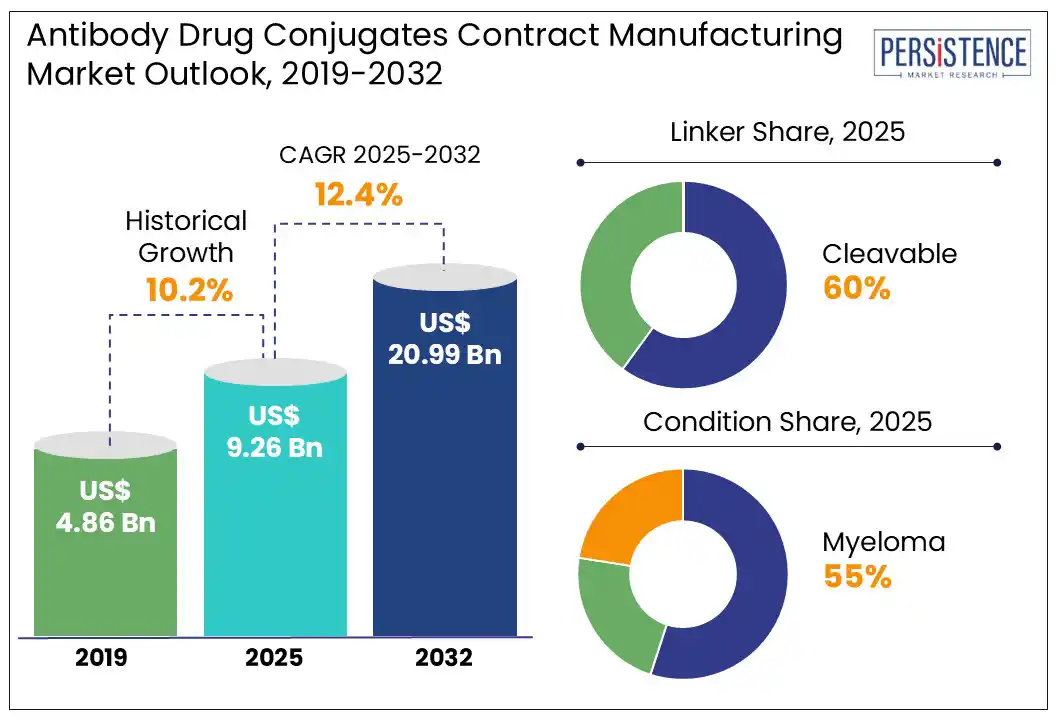
The global antibody drug conjugates contract manufacturing market size is likely to be valued at US$ 9.26 bn in 2025 and is estimated to reach US$ 20.99 bn in 2032, at a CAGR of 12.4% during the forecast period 2025 – 2032.
According to the Persistence Market Research report, market growth is driven by increasing incidence of cancer cases across the globe, high demand for antibody drug conjugates (ADCs) in cancer treatment, and challenges associated with developing ADCs.
ADCs are targeted medicines that deliver chemotherapy agents directly to cancer cells via a linker attached to a monoclonal antibody. Unlike conventional chemotherapy, ADCs deliver a drug directly into cancer cells and minimize damage to healthy ones, thereby improving treatment effectiveness and reducing side effects compared to traditional chemotherapy. By partnering with experienced Contract Development and Manufacturing Organization (CDMOs) and Contract Manufacturing Organization (CMOs) that offer integrated solutions that streamline development, reduce the time-to-market, and eliminate the need for significant in-house investments, companies can focus on their core competencies. Lonza, Catalent, WuXi Biologics, and Samsung Biologics are some key CDMOs.
Key Industry Highlights
|
Global Market Attribute |
Key Insights |
|
Antibody Drug Conjugates Contract Manufacturing Market Size (2025E) |
US$ 9.26 Bn |
|
Market Value Forecast (2032F) |
US$ 20.99 Bn |
|
Projected Growth (CAGR 2025 to 2032) |
12.4% |
|
Historical Market Growth (CAGR 2019 to 2024) |
10.2% |
The global incidence of cancer has been steadily increasing. According to the data released by the International Agency for Research on Cancer (IARC), in 2022, there were an estimated 20 million new cancer cases and 9.7 million deaths. The estimated number of people who were alive within five years following a cancer diagnosis was 53.5 million. About 1 in 5 people develop cancer in their lifetime, and approximately 1 in 9 men and 1 in 12 women die from the disease. According to WHO 2025 data, risk factors such as tobacco use, high body mass index, alcohol consumption, poor diet, and physical inactivity contribute to roughly one-third of all cancer deaths. Factors such as geriatric populations, lifestyle changes, and environmental changes contribute to the growing cancer incidence, prompting pharmaceutical companies to accelerate ADC development.
As of 2024, there were over 700 million people aged 65 and older worldwide, with projections indicating this number will double by 2050. This demographic shift is particularly pronounced in countries such as Japan and South Korea, where nearly 40% of their populations will be aged over 65 by 2050. The increasing prevalence of age-related cancers highlights the need for targeted therapies such as ADCs, which offer precision treatment options. Due to the technical challenges, high costs, and specialized infrastructure required for ADC production, many drug developers are turning to CDMOs for development.
CDMOs must adhere to Good Manufacturing Practices (GMP) and comply with diverse regulatory frameworks across regions. In the EU, the European Medicines Agency (EMA) mandates compliance with Annex 1, requiring the use of Grade A isolators for handling cytotoxic ADC payloads. This regulation compels European CDMOs to retrofit facilities with barrier systems costing between US$2–4 mn per production line. The U.S. Food and Drug Administration (FDA) enforces current GMP (cGMP) under Title 21 CFR, emphasizing the need for comprehensive quality systems and documentation. According to the FDA, outsourcing manufacturing does not excuse sponsors of product quality, highlighting the shared accountability between sponsors and CMOs/CDMOs.
ADC manufacturing involves handling potent substances, classified under Occupational Exposure Band (OEB) 4, necessitating specialized containment and safety measures to protect personnel and prevent cross-contamination. These regulatory requirements require CDMOs to incur significant investments in infrastructure, training, and quality systems. One notable example of FDA declining approval is patritumab deruxtecan, developed by Merck and Daiichi Sankyo for treating non-small cell lung cancer with EGFR mutations.
Personalized & precision medicine has become central to oncology treatment strategies. Increasing R&D efforts in developing patient-specific therapies demand highly specialized manufacturing processes capable of producing tailored ADCs with strict quality control. For example, ImmunoGen, now a part of AbbVie, collaborated with multiple CDMOs such as Lonza, to leverage their advanced bioconjugation technologies and flexible manufacturing capabilities to accelerate the development of their personalized ADC candidates. Another case is Seagen (formerly Seattle Genetics), which has partnered with Catalent for scalable manufacturing of their ADCs such as Adcetris® (brentuximab vedotin), used in targeted lymphoma treatments.
These collaborations illustrate how the surge in personalized medicine drives the demand for innovative contract manufacturing solutions that can meet stringent regulatory standards while enabling faster time-to-market for patient-specific therapies. Daiichi Sankyo and AstraZeneca developed Enhertu® (Trastuzumab deruxtecan) through Lonza, providing services such as bioconjugation and aseptic filling. In 2023, Lonza announced the expansion of its bioconjugation capacity by adding two new bioconjugation suites at its site in Visp, Switzerland, expected to be operational by 2026.
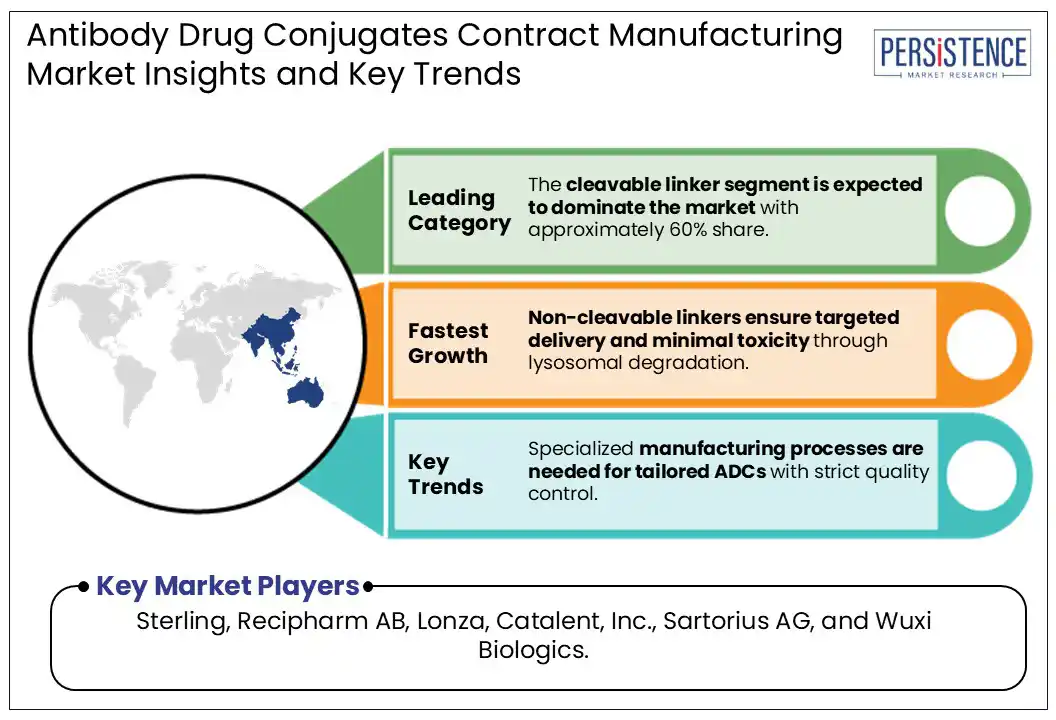
By linker, the cleavable linker segment is expected to dominate the market, holding approximately 60% of the market share during the forecast period. ADCs are composed of a drug (payload) and an antibody (mAbs) that are bound using linkages that are specifically designed to deliver the payload to the intended target environment sparing healthy cells, thereby targeting specific cancer types. Cleavable linkers use the inherent properties of tumor cells for the selective release of payloads from the ADCs. Cleavable linkers may also help with destroying cancer cells adjacent to their targets through the bystander effect. Adcetris® (brentuximab vedotin) uses a Val-Cit linker to connect monomethyl auristatin E (MMAE) to an anti-CD30 monoclonal antibody. After internalization, cathepsin B cleaves the linker, releasing MMAE.
The non-cleavable linker segment is expected to experience the fastest growth over the forecast period. A non-cleavable linker in an ADC is a chemically stable bond that remains intact even after the ADC is internalized by a cancer cell, releasing the cytotoxic payload only after the antibody is fully degraded within the lysosome. Non-cleavable linkers offer high stability in systemic circulation, minimizing off-target toxicity. Payload release occurs only after lysosomal degradation of the ADC, ensuring site-specific action.
Upon degradation, the cytotoxic drug is released along with part of the linker and possibly an amino acid, forming a modified but active compound. This design tends to produce a lower bystander effect since the released drug is often polar and less permeable to neighboring healthy cells. An example is Kadcyla® (ado-trastuzumab emtansine), which uses a non-cleavable thioether linker (SMCC) to attach the cytotoxic agent DM1 to the HER2-targeting antibody trastuzumab.
By condition, the myeloma segment is expected to dominate the market in 2025, accounting for around 55% of total revenue. Multiple myeloma accounts for 10% of all hematological malignancies. Multiple myeloma, a malignancy of plasma cells, often becomes resistant to conventional therapies such as proteasome inhibitors and immunomodulators. Myeloma is an important hematological malignancy in older adults, with a relatively poor prognosis.
According to a May 2025 National Library of Medicine study, globally, 188, 000 myeloma cases and 121 000 deaths were estimated in 2022. ADCs target surface antigens such as BCMA (B-cell maturation antigen) and deliver cytotoxic agents directly to cancer cells, minimizing harm to healthy tissues. A leading example is Blenrep® (belantamab mafodotin), developed by GSK, which became the first FDA-approved ADC for relapsed/refractory multiple myeloma.
The lymphoma segment is anticipated to be the fastest-growing segment within this market, driven by the increasing adoption of ADCs in lymphoma treatment protocols. The connection between lymphoma and ADC contract manufacturing is increasingly significant due to the expanding role of ADCs in treating various lymphoma subtypes, including diffuse large B-cell lymphoma (DLBCL), Hodgkin lymphoma, and follicular lymphoma. ADCs combine monoclonal antibodies with cytotoxic agents, enabling targeted delivery of chemotherapy to cancer cells while minimizing damage to healthy tissues.
Avid Bioservices partnered with ADC Therapeutics for the commercial production of Zynlonta (loncastuximab tesirine), an ADC targeting CD19-positive B-cell malignancies, including DLBCL. Avid's Tustin, California, facility is equipped with single-use bioreactors and quality control labs to meet the complex manufacturing requirements of ADCs.
Asia Pacific is estimated to dominate the antibody drug conjugates contract manufacturing market in 2025, accounting for a market share of approximately 45% over the forecast period, driven regulatory support, cost advantages, and a robust manufacturing ecosystem. Korea, China, and Taiwan are members of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), which facilitates the alignment of regulatory standards with global norms, simplifying the approval process for ADCs. The region offers cost-effective manufacturing solutions, with countries including India and South Korea providing competitive pricing for ADC production. AstraZeneca's US$1.5 bn ADC facility in Singapore is backed by the country's Economic Development Board.
China is emerging as a key player in the global antibody drug conjugate contract manufacturing market. The National Medical Products Administration (NMPA) has implemented expedited approval processes for innovative therapies, including ADCs, to accelerate their availability in the market. Additionally, the Made in China 2025 initiative aims to enhance the biopharmaceutical sector by reducing reliance on foreign technology. Companies, including WuXi Biologics have established state-of-the-art facilities in cities such as Shanghai, Wuxi, and Hangzhou, equipped with large-scale bioreactor capacities and dedicated lines for ADC production, adhering to cGMP standards. In 2024, Yilian Bio, a Chinese biotech company, entered into an out-licensing agreement with Roche to develop the next-generation ADC candidate YL211 to treat solid tumors.
North America is also expected to witness the fastest-growth during the forecast period. The U.S. leads the region due to early adoption of ADCs, multiple FDA approvals (Blenrep and Enhertu), and robust R&D activities. Companies including Lonza, Catalent, WuXi Biologics, and Merck are investing in advanced bioconjugation technologies and expanding their capacity for GMP-compliant ADC production. Canada is also witnessing growth due to government-backed research collaborations and the rising adoption of precision medicine. The region is supported by FDA and has a strong pipeline of ADCs targeting cancers such as myeloma and lymphoma. In August 2023, Pfizer announced a major investment to expand its biomanufacturing site in Kalamazoo, Michigan.
The U.S. is poised for continued growth. The U.S. is experiencing robust growth, fueled by the rising demand for targeted cancer therapies, technological advancements, and significant strategic investments. According to the National Cancer Institute website, in 2025, an estimated 2,041,910 new cases of cancer will be diagnosed in the U.S., and 618,120 people will die from the disease. Regulatory support from the FDA, which has approved multiple ADCs for cancer treatment, is accelerating market expansion. Major players such as Merck and MilliporeSigma are investing heavily, including Merck’s US$59 mn investment in its Wisconsin facility to boost ADC and highly potent active pharmaceutical ingredient (HPAPI) production.
Europe is witnessing moderate growth, propelled by technological advancements in automation, AI integration, and sustainability initiatives, alongside a robust biopharmaceutical infrastructure. The oncology segment, particularly myeloma and lymphoma therapies, remains a key revenue driver. Regulatory support across Germany, Switzerland, and the UK, combined with strong manufacturing capabilities, makes Europe an attractive hub for ADC production. The market is bolstered by strategic investments and collaborations, such as the US$11 bn Bristol Myers Squibb–BioNTech partnership for bispecific ADC development.
Germany is emerging as a key hub. The country leads the region in technological innovation, particularly in scalable manufacturing solutions such as single-use reactors and site-specific conjugation methods. Major players such as BioNTech, Lonza, and Sartorius drive expansion through strategic investments and partnerships. Germany’s strong regulatory framework, advanced pharmaceutical infrastructure, and growing focus on precision oncology position it as a central player in the global ADC landscape.
The global antibody drug conjugates contract manufacturing market is highly competitive, with global and domestic players offering a wide range of products and competing for a higher market share. Companies are investing in R&D and adopting growth strategies such as product innovations, strategic partnerships, and acquisitions.
The global market is projected to be valued at US$ 9.26 bn in 2025.
Market growth is driven by rising global cancer incidence, high demand for ADCs in cancer treatments, and the challenges associated with developing ADCs.
The market is poised to witness a CAGR of 12.4% from 2025 to 2032.
Increasing R&D efforts in developing patient-specific therapies demand highly specialized manufacturing processes capable of producing tailored ADCs with strict quality control.
Major players in the Global Antibody Drug Conjugates Contract Manufacturing Market include Sterling, Recipharm AB, Lonza, Catalent, Inc., Sartorius AG, and Wuxi Biologics.
|
Report Attribute |
Details |
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis |
Value: US$ Bn |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
|
Customization and Pricing |
Available upon request |
By Linker
By Condition
By Phase
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author