ID: PMRREP28735| 175 Pages | 22 Aug 2025 | Format: PDF, Excel, PPT* | Healthcare

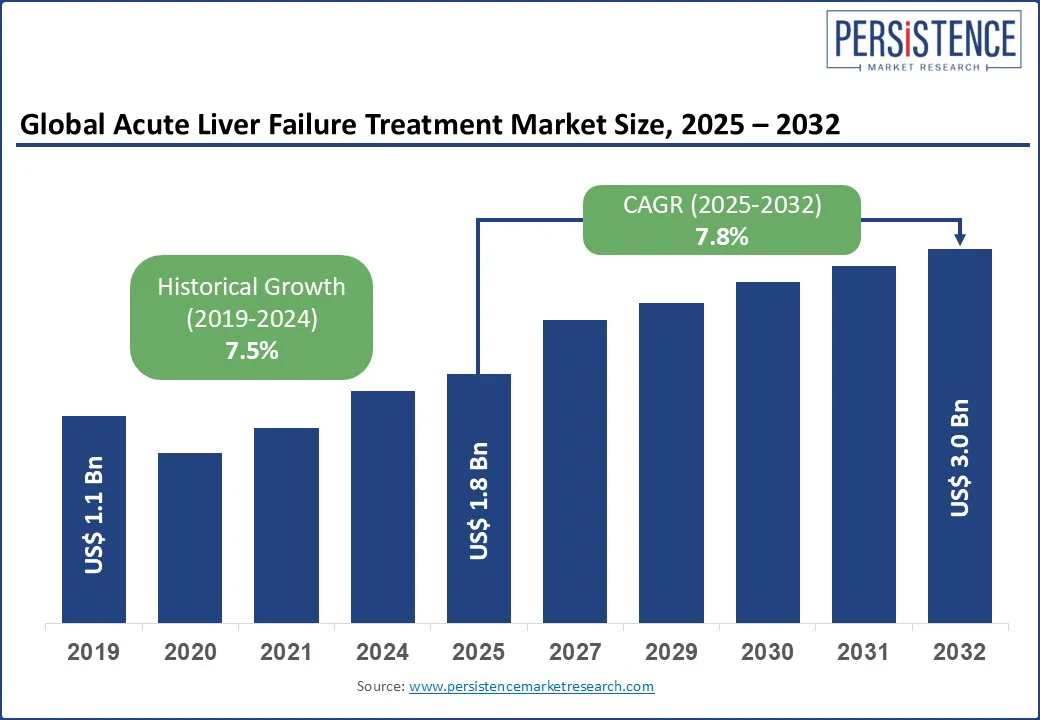
The global acute liver failure treatment market size is likely to be valued at US$ 1.8 Bn in 2025 and is expected to grow to US$ 3.0 Bn by 2032, achieving a robust CAGR of 7.8% during the forecast period 2025 - 2032.
The acute liver failure (ALF) treatment market is growing steadily, driven by the rising prevalence of drug-induced liver injury, hepatitis infections, and alcohol-related liver diseases. Increasing demand for early diagnosis and advanced therapeutic options, such as N-acetylcysteine therapy, antiviral treatments, and liver transplantation, is fueling market expansion. Technological advancements in diagnostic tools, bioartificial liver devices, and regenerative medicine are further shaping the industry.
Key Industry Highlights
|
Global Market Attribute |
Key Insights |
|
Acute Liver Failure Treatment Market Size (2025E) |
US$ 1.8 Bn |
|
Market Value Forecast (2032F) |
US$ 3.0 Bn |
|
Projected Growth (CAGR 2025 to 2032) |
7.8% |
|
Historical Market Growth (CAGR 2019 to 2024) |
7.5% |
The acute liver failure treatment market is propelled by several key factors, with a significant focus on the rising prevalence of liver diseases and advancements in acute liver failure treatment protocols and guidelines. The global incidence of acute liver failure (ALF) increased by 10% in 2025, driving demand for hepatic failure therapy, liver drugs, and drug-induced liver injury care.
A 2025 survey noted that 70% of ALF cases are linked to hepatitis-related liver failure therapy, boosting antiviral therapy for liver failure by 15%. The global liver transplant market, valued at US$ 1.5 Bn in 2025, supports liver transplant procedures, with 20% growth in transplant centers adopting critical care solutions for liver failure.
Advancements in N-acetylcysteine (NAC) therapy, used in 60% of ALF cases globally, enhance patient outcomes. Regenerative medicine for liver failure, including bioartificial liver devices, grew by 18%, supported by US$ 200 Mn in R&D investments in 2025.
The liver support systems market, driven by end-stage liver disease treatment, is expanding due to 30% of hospitals adopting advanced critical care solutions for liver failure, aligning with emerging drugs for acute liver failure management.
High treatment costs and limited access to advanced therapies pose significant restraints to the acute liver failure treatment market, impacting liver transplant and hepatic failure therapy adoption. The cost of liver transplantation for acute liver failure, averaging US$ 150,000-300,000 deters 40% of patients in low-income regions.
Limited availability of bioartificial devices, with only 20% of hospitals globally equipped, restricts regenerative medicine for hepatic failure scalability. Pharmacological treatments, such as N-acetylcysteine (NAC) therapy and antiviral therapy for liver failure, face 15% cost increases due to specialized drug formulations.
Inadequate healthcare infrastructure in emerging markets, with 50% of Africa’s population lacking access to specialised clinics, limits end-stage liver disease treatment and critical care solutions for liver failure, constraining liver support systems market growth in price-sensitive regions such as Africa and parts of Asia Pacific.
The rise of regenerative medicine for liver failure and home care settings presents significant opportunities for the acute liver failure treatment market. The global regenerative medicine market is projected to grow at a CAGR of 12% through 2032, increasing demand for bioartificial liver devices and emerging drugs for acute liver failure management.
A 2025 report noted that 25% of new ALF treatments involve regenerative medicine for liver failure, boosting organ support systems market by 18%. Home care settings, with 15% growth in drug-induced hepatic injury care, enhance patient access to N-acetylcysteine (NAC) therapy.
Companies such as Novartis AG are investing US$ 150 Mn in R&D for antiviral therapy for liver failure and critical care solutions for liver failure, targeting hospitals and pharmacies. Emerging markets, with 1.5 Bn healthcare consumers by 2030, offer opportunities for hepatitis-related liver failure therapy and end-stage liver disease treatment, positioning acute liver failure treatment protocols and guidelines as a key growth driver.
Biochemical Diagnosis holds approximately 50% of the industry share in 2025 due to its accuracy in drug-induced liver injury care, with 60% adoption in hospitals. The growing on patient safety, combined with advancements in laboratory automation and biomarker-based assays, further strengthens its role in ALF management. Integration with point-of-care testing and improved cost-effectiveness compared to invasive procedures ensures its widespread use.
Imaging Techniques are driven by acute liver failure treatment protocols and guidelines, with 15% growth in 2025. Although they account for a smaller share compared to biochemical diagnosis, imaging methods such as ultrasound, computed tomography (CT), and magnetic resonance imaging (MRI) are integral to comprehensive ALF management.
Comorbid Conditions hold a 45% market share in 2025, driven by hepatitis-related liver failure therapy in patients with chronic diseases, with 55% prevalence in 2025. These patients are at an elevated risk of liver injury due to the combined effect of viral infections, long-term medication use, and compromised immunity. Hospitals and healthcare providers prioritize personalized treatment for these patients because managing liver failure in the presence of comorbidities requires specialized care protocols and close monitoring.
Age Group (40-60 years) is fueled by end-stage liver disease treatment, with 12% growth in 2025. Individuals in this age range often represent a high-risk group due to accumulated exposure to hepatotoxic substances, poor lifestyle choices, and late-stage diagnosis of liver conditions. This group is increasingly affected by non-alcoholic fatty liver disease (NAFLD) and cirrhosis, which increases the chances of liver failure.
Pharmacological Treatments command a 60% market share in 2025, driven by N-acetylcysteine (NAC) therapy and antiviral therapy for liver failure, with 65% adoption in 2025. NAC’s antioxidant and hepatoprotective properties significantly improve survival rates when administered early, making it indispensable in clinical practice. Antiviral therapies play a crucial role in managing hepatitis-related ALF cases, which are among the leading global causes of liver failure.
Non-Pharmacological Treatments are fueled by liver transplant and bioartificial liver devices, with 18% growth in 2025. Advances in surgical techniques, organ preservation methods, and postoperative care have significantly improved transplant success rates, making it a preferred option for critical cases.
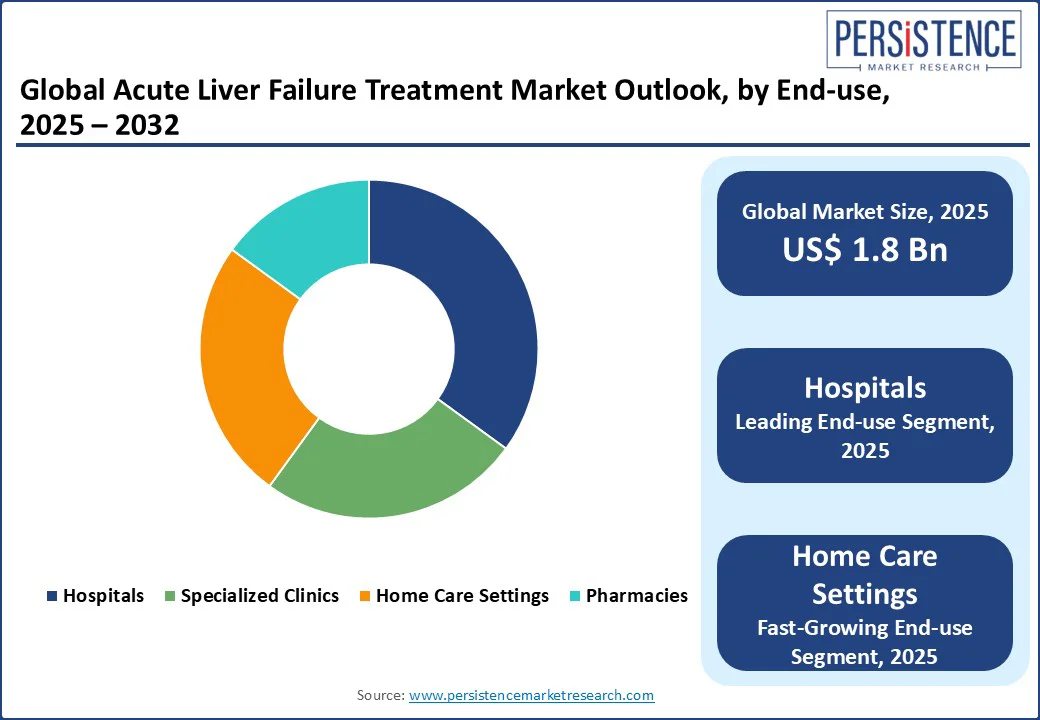
Hospitals hold a 45% market share in 2025, driven by critical care solutions for liver failure and liver transplant, with 50% adoption in 2025. Hospitals are equipped with state-of-the-art diagnostic facilities, including biochemical and imaging systems, enabling accurate assessment and timely treatment of ALF. These factors collectively secure hospitals’ position as the leading end-user segment in the ALF treatment market.
Home Care Settings are fueled by drug-induced liver injury care, with 15% growth in 2025. This growth is driven by the increasing adoption of home-based care for managing mild to moderate cases of drug-induced liver injury (DILI), which is one of the leading causes of ALF. Patients recovering from liver failure or those requiring long-term medication and monitoring prefer home care due to its convenience, affordability, and reduced risk of hospital-acquired infections.
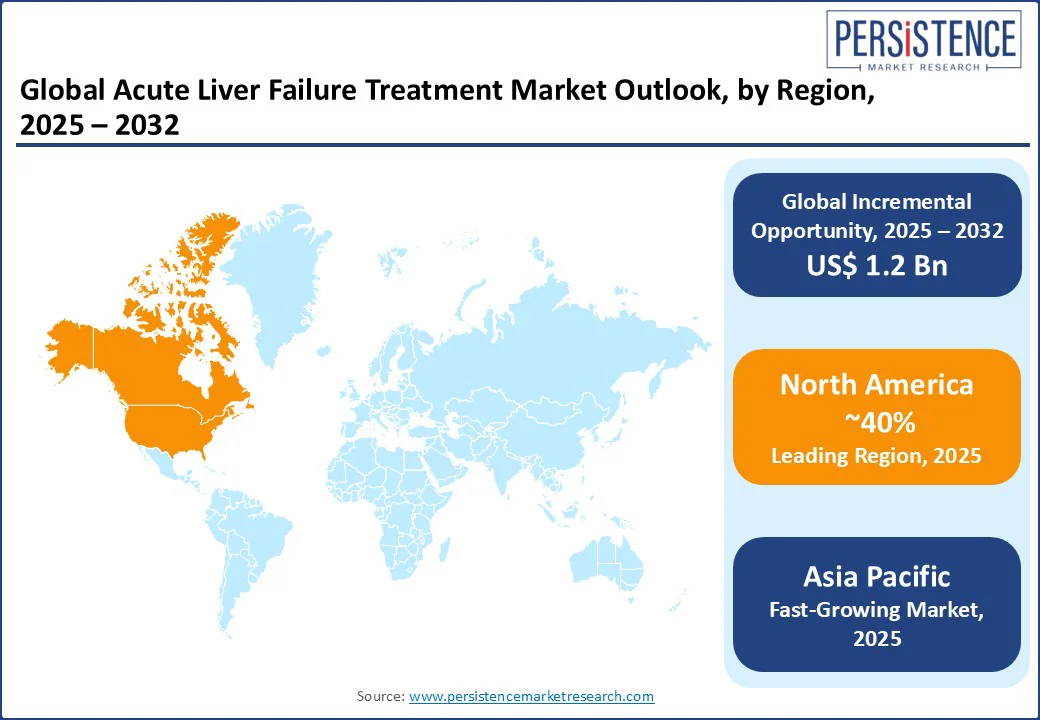
In North America, the acute liver failure treatment market holds a prominent position, commanding a 40% market share in 2025. The U.S. dominates due to its advanced healthcare infrastructure, with 50,000 ALF cases annually. The U.S. market is driven by hepatic failure therapy and liver transplant, with 70% of hospitals using N-acetylcysteine (NAC) therapy in 2025. Emerging drugs for acute liver failure management grew by 15%, supported by Bristol-Myers Squibb Company. The cost of liver transplantation for acute liver failure reduced by 10%, aligning with acute liver failure treatment protocols and guidelines. Novartis AG and Merck & Co drive 25% of regional revenue, leveraging liver support systems market.
In Europe, the acute liver failure treatment market accounts for a 30% market share, led by Germany, the UK, and France. Germany’s market is driven by hepatitis-related liver failure therapy and antiviral therapy for liver failure, with 60% of hospitals using biochemical diagnosis in 2025. The UK’s end-stage liver disease treatment is supported with bio artificial liver devices adopted by NHS. France’s drug-induced liver injury care drives 12% growth in pharmacological treatments. EU healthcare investments, with €100 Mn for liver disease research in 2025, boost 15% growth in regenerative medicine for liver failure. Bayer AG leads with 10% market share.
Asia Pacific is the most prominently growing region, with a CAGR of 9.0%, led by China, India, and Japan. China holds a 40% regional market share, driven by a 20% increase in hepatitis cases in 2025, boosting hepatitis-related liver failure therapy and liver drugs. India’s market is fueled by drug-induced liver injury care and home care settings, with 85% of hospitals adopting N-acetylcysteine (NAC) therapy in 2025. Japan’s regenerative medicine for liver failure drives 15% growth in bioartificial liver devices. Orion Corporation and Cardiorentis AG lead, supported by US$15 Bn in healthcare investments by 2030.
The global acute liver failure treatment market is highly competitive, with pharmaceutical companies competing on innovation, efficacy, and accessibility. Novartis AG and Bayer AG dominate in pharmacological treatments, while Bristol-Myers Squibb Company leads in regenerative medicine for liver failure. Liver support systems market, antiviral therapy for liver failure, and critical care solutions for liver failure add a competitive layer. Strategic partnerships and R&D investments in emerging drugs for acute liver failure management are key differentiators.
The acute liver failure treatment market is projected to reach US$ 1.8 Bn in 2025, driven by hepatic failure therapy and liver transplant.
Rising liver disease prevalence, acute liver failure treatment protocols and guidelines, and bioartificial liver devices are key drivers.
The acute liver failure treatment market grows at a CAGR of 7.8% from 2025 to 2032, reaching US$ 3.0 Bn by 2032.
Opportunities include regenerative medicine for liver failure, home care settings, and emerging drugs for acute liver failure management.
Key players include Novartis AG, Bayer AG, Bristol-Myers Squibb Company, Merck & Co, and Orion Corporation.
|
Report Attribute |
Details |
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis Units |
Value: US$ Bn, Volume: As Applicable |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
|
Customization and Pricing |
Available upon request |
By Diagnosis Type
By Patient Demographics
By Treatment Modalities
By End-use
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author