ID: PMRREP29491| 192 Pages | 26 Jun 2025 | Format: PDF, Excel, PPT* | Healthcare

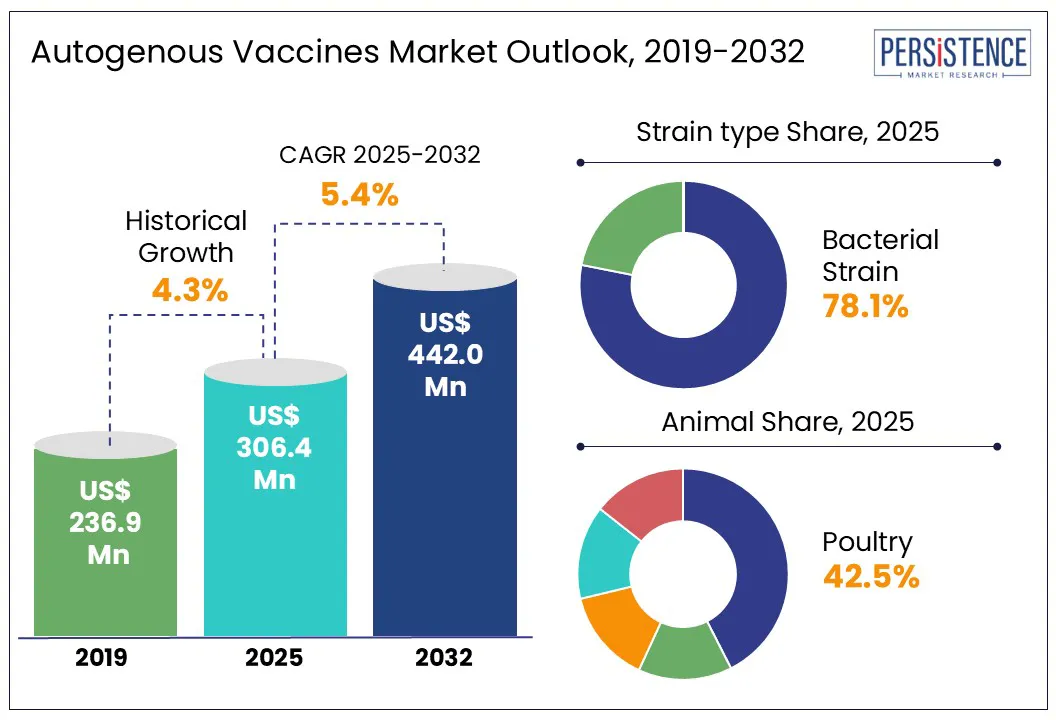
According to the Persistence Market Research report, the global autogenous vaccines market size is likely to be valued at US$ 306.4 Mn in 2025 and is expected to reach US$ 442.0 Mn by 2032, growing at a CAGR of 5.4% during the forecast period from 2025 to 2032.
Autogenous vaccines, tailored to specific pathogens from individual herds or flocks, play a crucial role in targeted disease prevention, particularly when commercial vaccines fall short. Key market trends include advancements in diagnostic and vaccine production technologies, a shift toward precision livestock farming, and growing awareness of antimicrobial resistance.
Strategic partnerships are also bolstering market growth. For instance, in August 2024, Merck Animal Health in partnership with Cambridge Technologies received USDA (U.S. Department of Agriculture) approval for an experimental autogenous vaccine for avian metapneumovirus type B, which poses a significant threat to broilers, broiler breeders, layers, and turkey breeders. Evolving livestock management practices, the need for enhanced biosecurity, and rising investments in animal health further support the market.
Key Industry Highlights:
|
Global Market Attribute |
Key Insights |
|
Autogenous Vaccines Market Size (2025E) |
US$ 292.1 Mn |
|
Market Value Forecast (2032F) |
US$ 306.4 Mn |
|
Projected Growth (CAGR 2025 to 2032) |
5.4% |
|
Historical Market Growth (CAGR 2019 to 2024) |
4.3% |
Commercial vaccines, while essential in animal disease prevention, present certain limitations that drive the demand for autogenous vaccines. As outlined in the 2023 HealthforAnimals report, many animal diseases continue to challenge veterinary medicine, with traditional vaccines often failing to offer timely or effective protection against rapidly evolving pathogens.
Most commercial vaccines are based on classical platforms, which, although reliable, lack the flexibility required for fast response to emerging or localized disease threats. They are generally not designed to target specific pathogen strains found within individual herds or regions, limiting their utility during localized outbreaks.
Autogenous vaccines provide a highly targeted solution, using pathogens isolated from the affected animals to create a customized immunization. This precision approach proves invaluable when commercial options are unavailable or inadequate. In December 2024, over 6 million U.S. poultry received an experimental autogenous vaccine for avian metapneumovirus (aMPV) type B developed by Merck Animal Health and Cambridge Technologies—demonstrating its value in emergency disease control. This growing reliance on autogenous vaccines highlights their critical role in enabling rapid, localized disease control.
The limited understanding of veterinary disease epidemiology, especially for region-specific and emerging pathogens is a key factor restraining the global autogenous vaccines market. Rapid genetic mutations, inadequate surveillance, and complex pathogen diversity hinder accurate identification of vaccine targets and slow the development of effective, herd-specific formulations. These challenges are exacerbated by weak collaboration between research institutions and vaccine manufacturers, poor insights into immune responses, and the absence of robust data-sharing systems.
Additionally, stringent regulatory requirements significantly impact the speed and cost of vaccine development. For example, under USDA APHIS Memorandum 800.69, autogenous vaccines must meet rigorous standards such as pathogen isolation, sterility testing, and inactivation validation, which restrict scalability and delay deployment. Their use is often limited to specific flocks or herds, with strict timeframes and mandatory retesting.
These regulatory and scientific constraints collectively limit the market’s ability to respond swiftly and effectively to emerging animal health threats. These multifaceted challenges underscore the need for stronger research frameworks, streamlined regulations, and enhanced industry collaboration to fully realize the potential of autogenous vaccines in global animal health.
Technological advancements present a significant opportunity for the autogenous vaccines market, enabling more efficient and tailored solutions for animal health. Innovations in diagnostic technologies, vaccine formulation, and production processes have enhanced the speed and accuracy of autogenous vaccine development. These advancements help meet the rising demand for customized vaccines, especially in scenarios where commercial vaccines are not effective or unavailable.
A notable example is Vaxxinova’s SRP® (Siderophore Receptor Protein) technology, which represents a breakthrough in bacterial vaccine development. Unlike traditional vaccines, SRP® technology targets iron receptors on the surface of bacteria, which are critical for bacterial survival. By neutralizing these receptors, SRP® vaccines help induce robust immunity in animals without the use of whole bacterial cells. This technology offers a safer and more effective vaccination strategy, particularly against complex bacterial infections in livestock.
Such advancements not only improve the safety and efficacy of autogenous vaccines but also support quicker vaccine production in response to emerging diseases. The integration of novel technologies like SRP® lead to more predictable outcomes, reduce the risk of adverse reactions, and enable a more strategic approach to disease management in animal husbandry.
Bacterial strain segment is projected to hold a revenue share of nearly 78% in 2025 within the global autogenous vaccines market.
Autogenous vaccines, also referred to as autovaccines, are formulated using bacterial or viral strains isolated directly from specific herds or farms, enabling highly targeted immunity against localized infections. These vaccines serve as vital alternatives when licensed or commercial options are unavailable or ineffective for specific pathogens.
The growing incidence of zoonotic disease outbreaks many of which are linked to multidrug-resistant bacterial strains in livestock has increased the urgency for effective non-antibiotic interventions. As antimicrobial resistance continues to rise, both regulatory authorities and livestock producers are prioritizing preventative strategies that reduce reliance on antibiotics. This shift is driving heightened demand for customized bacterial autogenous vaccines, reinforcing their role as a proactive and sustainable solution for disease management in modern animal health programs.
Poultry segment is expected to dominate the animal category in 2025 with nearly 42.5% of the global autogenous vaccines market share. The high demand for autogenous vaccines in the poultry sector is primarily driven by the industry's scale, the susceptibility of poultry to various infectious diseases, and the need for tailored vaccines to manage localized outbreaks.
The frequent emergence of diseases such as avian influenza, Newcastle disease, and other bacterial infections in poultry farms necessitates customized immunization solutions, boosting the adoption of autogenous vaccines in this segment. In 2023, the Animal and Plant Health Inspection Service (USDA), confirmed Highly Pathogenic Avian Influenza in commercial and backyard flocks, impacting over 378.5 million egg-laying chickens.
North America is anticipated to hold significant share of the global market in 2025, driven by advanced veterinary infrastructure, strong regulatory support, and a growing emphasis on animal health. The region's robust poultry industry, coupled with the rising threat of emerging diseases, fuels the demand for tailored vaccine solutions.
In September2024, Ceva Animal Health began antigen production for an experimental autogenous vaccine to combat the Avian Metapneumovirus (aMPV) outbreak, which caused significant losses in the U.S. poultry sector.
Additionally, in January 2025, the USDA's approved Vaxxinova's Vaxxon® SHS, the first live aMPV vaccine was launched in the U.S. Later in March 2025, Bimeda's aquaculture division, AquaTactics, received USDA approval to produce autogenous fish vaccines, thus, offering customized vaccine solutions nationwide, enhancing fish health and productivity across U.S. aquaculture operations. These advancements underscore North America's prominent role in advancing and implementing autogenous vaccine solutions.
Europe is estimated to lead the global market, accounting for nearly one-third of the total share in 2025, driven by a strong regulatory framework, evolving legislation, and proactive industry initiatives. The region's diverse legal landscape, as seen in countries such as Germany and the UK, highlights both challenges and opportunities for autogenous vaccine manufacturers.
The UK autogenous vaccines market benefits from advanced veterinary services, precision livestock farming, and growing farmer awareness around herd-specific health management. Notably, Mowi Scotland's 35% reduction in overall biomass mortality across all seawater farms in 2024, aided by autogenous vaccines from Ridgeway Biologicals, underscores their growing impact in aquaculture and livestock health, supporting sustainable disease management across diverse animal production sectors.
Germany autogenous vaccines market is fuelled by its strong veterinary infrastructure, strict antimicrobial resistance regulations, and early adoption of innovative solutions. The launch of the Dopharma-Ripac autogenous vaccine production facility in Potsdam in February 2025, exemplifies this growth, supported by active research collaborations and increasing farmer awareness of targeted, sustainable animal disease prevention.
Combined with rapid response capabilities during outbreaks and supportive legislation, Europe is well-positioned as a key global player in the autogenous vaccines sector.
The Asia Pacific region is anticipated to grow at a CAGR of 5.9% over the forecast period, supported by the region’s expanding livestock sector and rising demand for tailored disease management solutions.
Rising meat consumption particularly in China, which contributes over 28% of global meat production (Global Forest Coalition, 2022 report) is intensifying pressure on livestock systems and increasing the risk of disease outbreaks. Intensive farming practices, which place strain on forest ecosystems and animal health, are accelerating the adoption of autogenous vaccines for herd-specific disease control.
Recent developments across the region reflect this growing momentum. In March 2022, Apiam Animal Health initiated the development of Australia’s first autogenous viral vaccine facility, supported by the Victorian Government, to combat emerging diseases such as Japanese encephalitis and strengthen domestic vaccine production. Similarly, in July 2024, Barramundi Group partnered with UVAXX and Singapore’s A*STAR Infectious Diseases Labs to develop an epitope-based vaccine targeting Scale Drop Disease Virus (SDDV) in Asian sea bass, aiming to reduce stock losses and antimicrobial use. Collectively, these initiatives underscore Asia-Pacific’s rising role in driving innovation and expanding the autogenous vaccines market.
The global autogenous vaccine market features a competitive landscape marked by the presence of both global and regional players. Collaborations, acquisitions, and innovations in vaccine technology are common strategies adopted by market participants to strengthen their foothold. The market's competitive dynamics are also influenced by evolving regulatory frameworks, technological advancements, and the growing need for tailored vaccines to address specific animal health challenges.
The global market is set to reach US$ 306.4 Mn in 2025.
The global market is projected to record a CAGR of 5.4% during the forecast period from 2025 to 2032.
Growing demand for tailored autogenous vaccines in aquaculture and livestock, decline in antimicrobial use, and increasing focus on preventative measures for herd health are the key driving factors for the market growth.
Ceva, HIPRA, Vaxxinova, Phibro Animal Health Corporation, Lohmann Breeders, IDT Biologika, Boehringer Ingelheim International GmbH, and Elanco Animal Health are a few leading players.
Europe is projected to dominate the global market in 2025.
|
Report Attribute |
Details |
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis Units |
Value: US$ Mn/Bn |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
|
Customization and Pricing |
|
By Strain Type:
By Animal:
By End-use:
By Country:
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author