ID: PMRREP25999| 213 Pages | 10 Dec 2025 | Format: PDF, Excel, PPT* | Healthcare

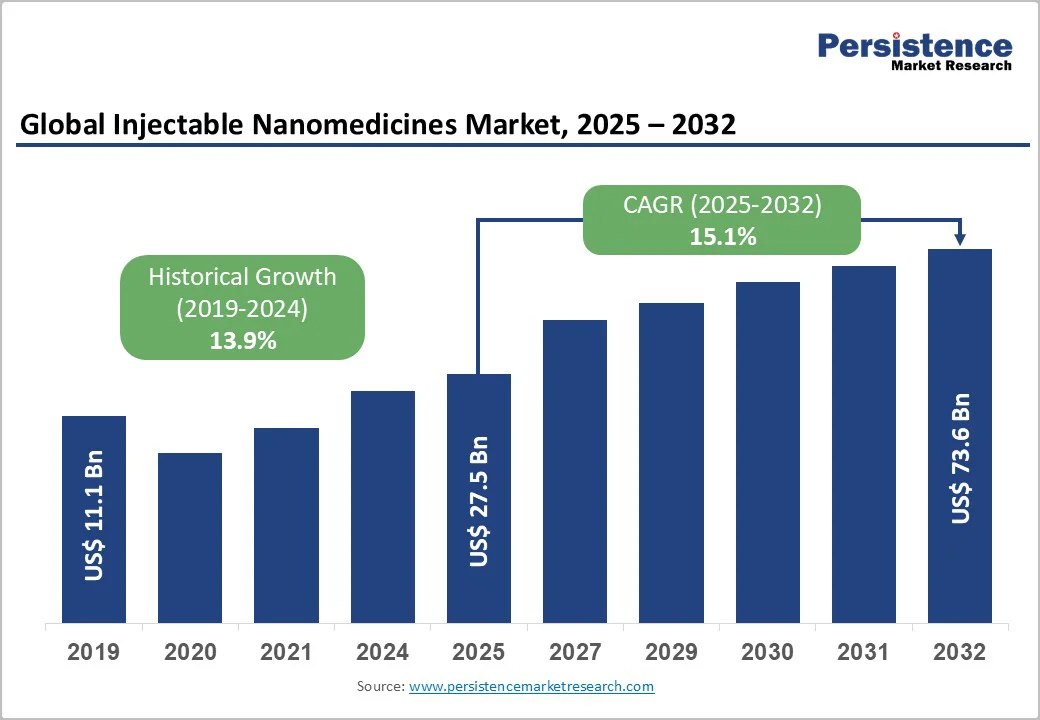
The global injectable nanomedicines market size is valued at US$27.5 billion in 2025 and is projected to reach US$73.6 billion by 2032, growing at a CAGR of 15.1% from 2025 to 2032.
The global injectable nanomedicines industry is growing steadily, driven by increasing surgical procedures, infection-control priorities, and a move toward single-use formats for improved safety and efficiency.
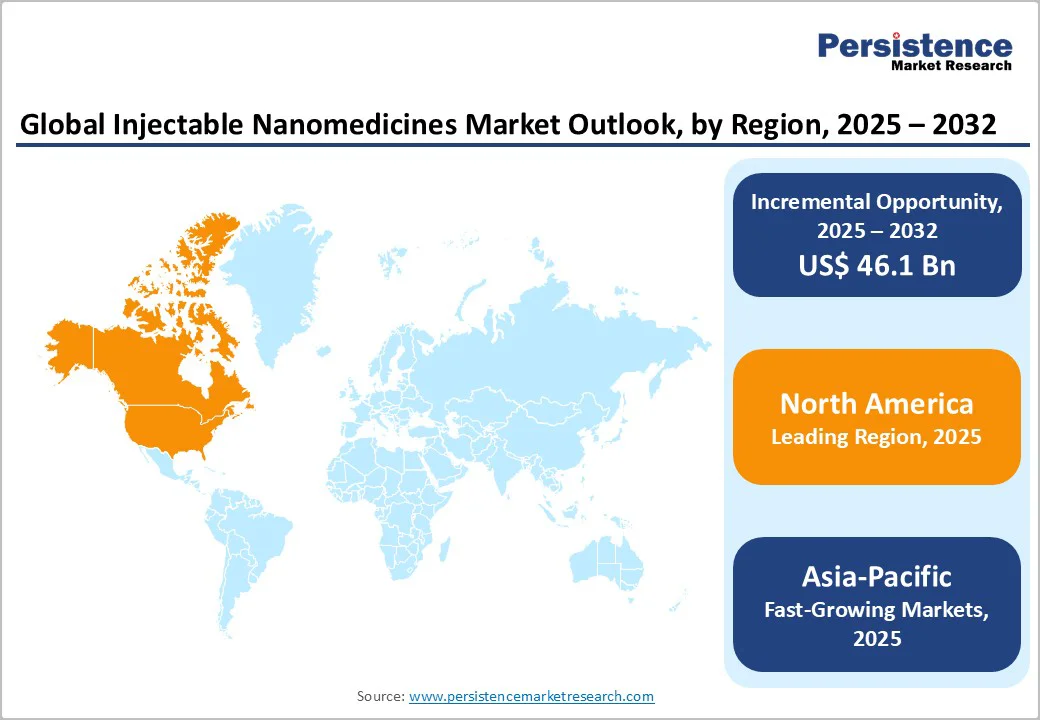
North America leads with advanced healthcare systems and robust regulatory support. At the same time, the Asia Pacific region records the fastest growth due to expanding hospital capacity, higher patient volumes, and the adoption of affordable disposable solutions.
| Key Insights | Details |
|---|---|
| Injectable Nanomedicines Market Size (2025E) | US$ 27.5 Bn |
| Market Value Forecast (2032F) | US$ 73.6 Bn |
| Projected Growth (CAGR 2025 to 2032) | 15.1% |
| Historical Market Growth (CAGR 2019 to 2024) | 13.9% |
The demand for advanced, targeted drug delivery systems in the Injectable Nanomedicine Market is supported by data from the US FDA and ClinicalTrials.gov. As of 2021, there were over 2,000 nanomedicine-related clinical trials globally, with more than 50% focused on drug delivery applications and approximately 40% targeting cancer treatment specifically.
The FDA has approved over 50 nanoparticle-based drug delivery products for clinical use, reflecting growing clinical adoption. Clinical outcomes further justify the market’s growth: certain nanoparticle-based cancer therapies have shown a 25% increase in 5-year survival rates versus traditional chemotherapy, and cancer patients demonstrate a 50% higher therapeutic response rate with nanomedicine compared to standard drugs.
These targeted systems significantly improve efficacy and reduce side effects, making them a primary growth driver supported by measurable clinical advancements and government-backed approvals, and data
High manufacturing complexity and cost are key barriers in bringing injectable nanomedicines to market, as underscored by FDA regulatory guidance and peer-reviewed studies. Nanomedicine formulation demands precise control of nanoscale features, such as particle size, charge, and morphology, necessitating advanced equipment and specialized processing expertise.
Ensuring batch-to-batch consistency and purity requires rigorous validation and extensive quality checks, often exceeding requirements for traditional drugs. According to the FDA, many manufacturers struggle with industrial-scale production, facing frequent scale-up failures and costly reproduction of lab results.
While over 100 nanomedicine-based formulations have been approved, the majority are produced by large pharma firms due to the steep investment required for process validation, equipment, and compliance. This challenge restricts smaller producers’ ability to compete, limits industry diversity, and increases the final cost for healthcare systems.
Personalized and precision medicine represents a major opportunity for the injectable nanomedicine market, with nanomedicines enabling highly individualized treatments for cancer, infectious, and rare diseases. As of 2021, over 2,000 nanomedicine-related clinical trials were registered globally, and approximately 40% were cancer-focused.
The U.S. FDA has approved more than 50 nanomedicine products, including tailored nanoparticle drug carriers designed for specific patient profiles, maximizing therapeutic benefit while minimizing systemic toxicity. Nanomedicine in cancer care has delivered a 45% increase in successful treatment rates and a 25% improvement in 5-year survival for certain cancers compared to traditional therapies.
Government funding for nanomedicine research in the U.S. reached $480 million in 2020, underscoring the high priority and promise of advancing these patient-specific interventions.
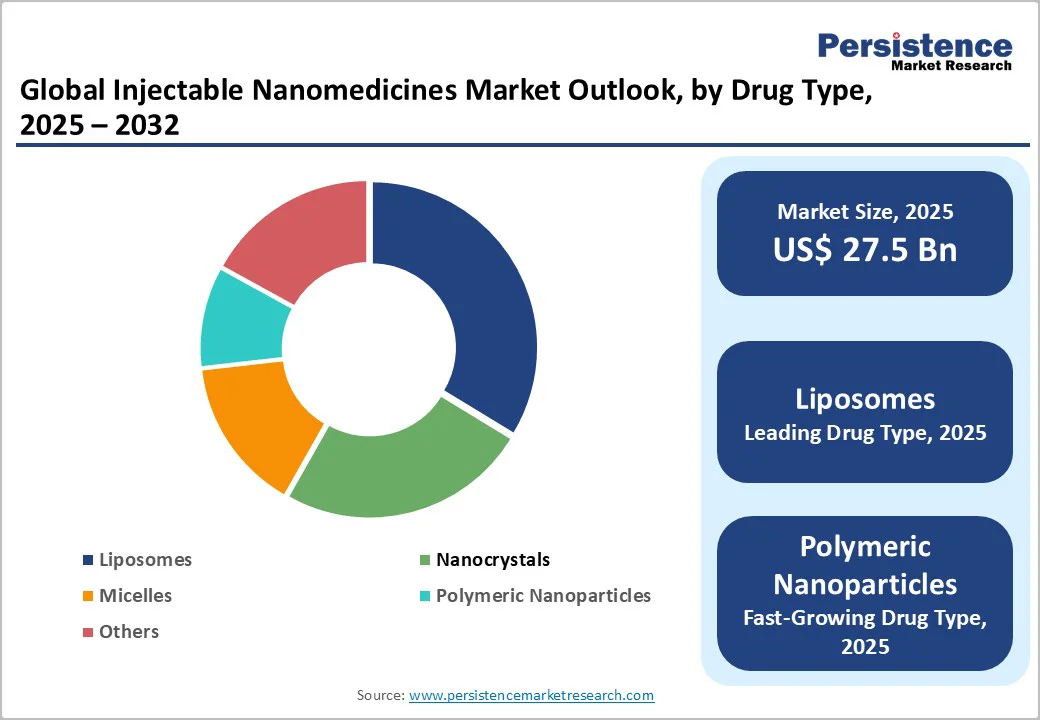
Liposomes occupy 33.7% share of the global market in 2025, due to their proven clinical effectiveness, biocompatibility, and regulatory approval track record. As of 2023, the FDA had approved 15 liposomal drug products, covering indications from cancer therapy (e.g., Doxil® for ovarian and breast cancer) to infections and pain management.
Liposomes’ unique phospholipid bilayer structure allows them to encapsulate both hydrophilic and hydrophobic drugs, improving circulation times and enabling targeted delivery to specific tissues while reducing toxicity-especially cardiotoxicity and other side effects.
In clinical use, targeted liposomal formulations have demonstrated 3-fold higher tumor cell uptake and over 88% greater cytotoxicity compared to free drugs, with extended patient survival. These advantages have cemented liposomes as the most successful and widely adopted injectable nanomedicine platform.
Cancer dominates the injectable nanomedicines market due to the urgent need for targeted, effective therapies that address the limitations of conventional cancer treatments. The US National Cancer Institute reports that several FDA-approved nanomedicines, including Doxil and Abraxane, have improved drug delivery efficiency and reduced systemic toxicity in cancer therapy.
For example, Doxil, the first FDA-approved liposomal doxorubicin, enhances drug accumulation in tumors while lowering side effects, increasing treatment tolerability. Abraxane, a nanoparticle albumin-bound paclitaxel, has expanded indications to breast, lung, and pancreatic cancers due to its enhanced efficacy.
These nanomedicines have shown improved pharmacokinetics, resulting in higher drug concentration within tumors and better patient outcomes. The FDA lists over a dozen nanotechnology-based cancer therapies, underscoring cancer’s dominant share in the injectable nanomedicine market due to its high prevalence and critical need for precision treatments.
North America dominates the injectable nanomedicines market with a 41.1% share in 2025, due to the National Nanotechnology Initiative (NNI), which has cumulatively invested over $45 billion since 2001 to advance nanoscale science and technology. In 2024 alone, the President’s budget allocated a record $2.16 billion towards NNI, emphasizing foundational and application-driven research.
This sustained investment supports advanced R&D, commercialization, and workforce development, fueling innovation in nanomedicines. Furthermore, multiple federal agencies, including NIH, FDA, and NSF, contribute substantial funding for nanotechnology health research, accelerating development and regulatory approvals.
The coordination and robust infrastructure resulting from NNI enable North America to maintain leadership in nanomedicine innovation, production, and clinical application, driving its dominance in the injectable nanomedicines market globally.
Europe is a leader in the injectable nanomedicines market due to robust public healthcare investments and strong research initiatives like Horizon Europe. These programs fund advanced nanomedicine research, fostering collaboration among universities, biotech startups, and pharmaceutical companies. Europe’s efficient regulatory frameworks support streamlined development and approvals, accelerating innovation.
Emphasis on personalized therapies, especially in oncology and rare diseases, drives demand for injectable nanomedicines. Collaborative projects such as ERA4Health further bolster research funding and international partnerships, enabling Europe to address public health needs with cutting-edge nanoscale technologies.
This ecosystem enhances Europe’s position in precision medicine and innovative drug delivery, making it a global frontrunner in nanomedicine development and commercialization
Asia Pacific is the fastest-growing region in the injectable nanomedicines market due to rising healthcare investments, a large geriatric population, and increasing chronic diseases like cancer and cardiovascular disorders. Countries such as China, India, Japan, and South Korea are leading in nanomedicine research and development, supported by government funding and supportive policies.
The region’s expanding healthcare infrastructure and growing awareness of advanced therapies drive market growth. Public initiatives and international collaborations further accelerate innovation and commercialization.
For instance, China hosts major nanotechnology conferences and invests heavily in industry hubs. The growing demand for life-saving drugs and advanced drug delivery systems, combined with increasing clinical trials and production capabilities, supports rapid market expansion in Asia Pacific.
Leading companies in the injectable nanomedicines market emphasize high-volume sterile production and material innovation. They invest in improved infection-prevention products, cost-efficient plastics, eco-friendly materials, and automated manufacturing lines.
Strategic partnerships with hospitals and distributors ensure reliable supply chains. Their R&D focuses on developing safer, stronger, and sustainable disposables to meet increasing surgical volumes and infection-control demands globally, thereby addressing expanding healthcare needs efficiently.
The global injectable nanomedicines market is valued at US$ 27.5 Bn in 2025.
Rise in the prevalence of chronic diseases, advancements in targeted drug delivery, growing healthcare investments, and strong R&D funding drive the injectable nanomedicine market expansion globally.
The global injectable nanomedicines market is poised to witness a CAGR of 15.1% between 2025 and 2032.
Key market opportunities include expanding personalized therapies, advancements in nanocarriers, smart theranostics, regenerative medicine growth, and emerging market commercialization.
Pacira Pharmaceuticals, Hospira (Pfizer), Novartis, Takeda Pharmaceuticals, Hikma Pharmaceuticals, Johnson & Johnson.
| Report Attribute | Details |
|---|---|
| Historical Data/Actuals | 2019 - 2024 |
| Forecast Period | 2025 - 2032 |
| Market Analysis | Value: US$ Bn |
| Geographical Coverage |
|
| Segmental Coverage |
|
| Competitive Analysis |
|
| Report Highlights |
|
By Drug Type
By Application
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author