ID: PMRREP13594| 197 Pages | 26 Nov 2025 | Format: PDF, Excel, PPT* | Healthcare

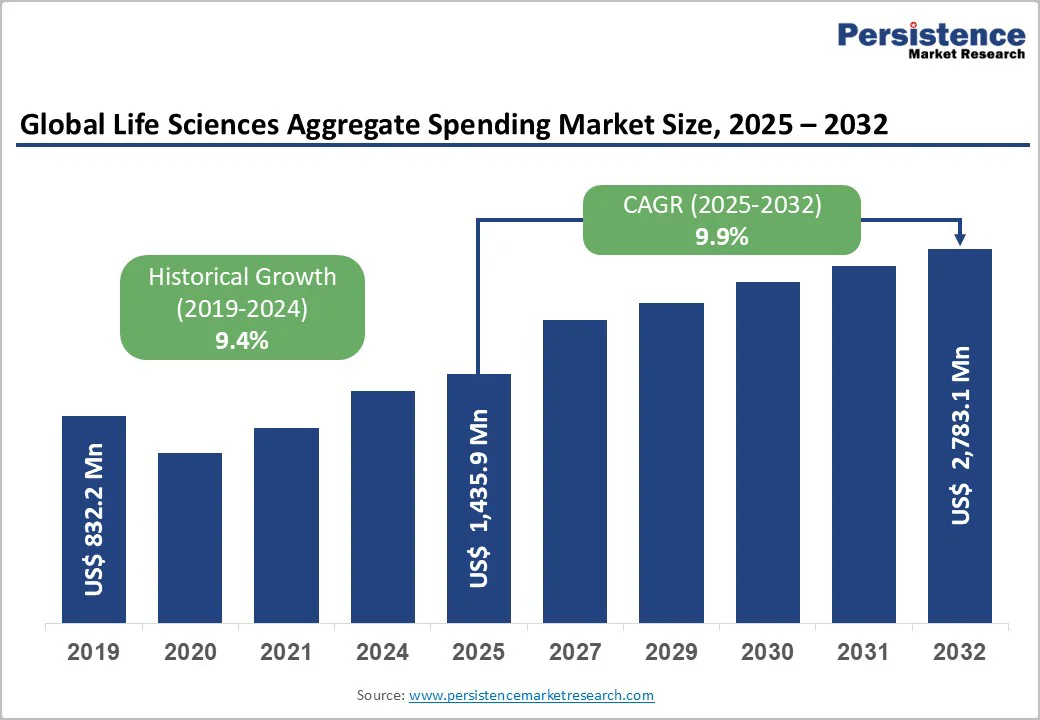
The global life sciences aggregate spending market size is valued at US$1,435.9 million in 2025 and is projected to reach US$2,783.1 million at a CAGR of 9.9% during the forecast period from 2025 to 2032.
The life sciences aggregate spending market focuses on solutions that enable pharmaceutical, biotechnology, and medical device companies to track and report financial interactions with healthcare professionals and organizations, ensuring transparency and regulatory compliance. Rising global scrutiny under frameworks such as the U.S. Sunshine Act and the EFPIA Disclosure Code has accelerated the adoption of automated, cloud-based reporting platforms.
| Key Insights | Details |
|---|---|
|
Life Sciences Aggregate Spending Market Size (2025E) |
US$ 1,435.9 Mn |
|
Market Value Forecast (2032F) |
US$ 2,783.1 Mn |
|
Projected Growth (CAGR 2025 to 2032) |
9.9% |
|
Historical Market Growth (CAGR 2019 to 2024) |
9.4% |
The growing number of micro, small, and medium enterprises (MSMEs) in the healthcare and life sciences industry is emerging as a powerful driver of aggregate spending. These smaller firms, often engaged in niche drug manufacturing, medical devices, and biotech innovation, face increasing regulatory obligations similar to large corporations but lack an in-house compliance infrastructure. As they scale operations and engage more frequently with healthcare professionals, distributors, and research partners, their transactional complexity multiplies. To remain audit-ready and transparent, MSMEs are rapidly adopting cost-effective, cloud-based aggregate spend solutions that automate data collection, validation, and disclosure.
A major restraint in the Life Sciences Aggregate Spending Market is the lack of standardized and structured physician databases across regions. Many healthcare systems, especially in emerging markets, do not maintain unified identifiers for healthcare professionals, making it difficult to map transactions and disclosures accurately. Inconsistent physician names, addresses, and affiliations lead to duplicate or mismatched records, undermining data integrity in compliance reports. Moreover, variations in data privacy regulations further limit access to comprehensive physician information. This fragmentation complicates payment tracking, delays report validation, and increases compliance risks for pharmaceutical and medical device companies striving to meet global transparency mandates. Establishing standardized healthcare provider registries is therefore essential to strengthen accuracy, accountability, and cross-border compliance efficiency.
The growing globalization of the life sciences sector has created strong demand for Unified Global Compliance Platforms that can consolidate financial transparency across multiple jurisdictions. Multinational pharmaceutical and medical device firms operate in regions with diverse regulations, reporting timelines, and disclosure formats, making compliance management highly complex. A single, unified dashboard that integrates multi-country, multi-language, and multi-currency capabilities enables seamless aggregation and harmonization of spend data. These platforms reduce redundancy, minimize regional reporting errors, and enable real-time visibility of global healthcare professional interactions. As compliance evolves into a strategic function, unified systems offer scalability, audit readiness, and a true competitive edge.
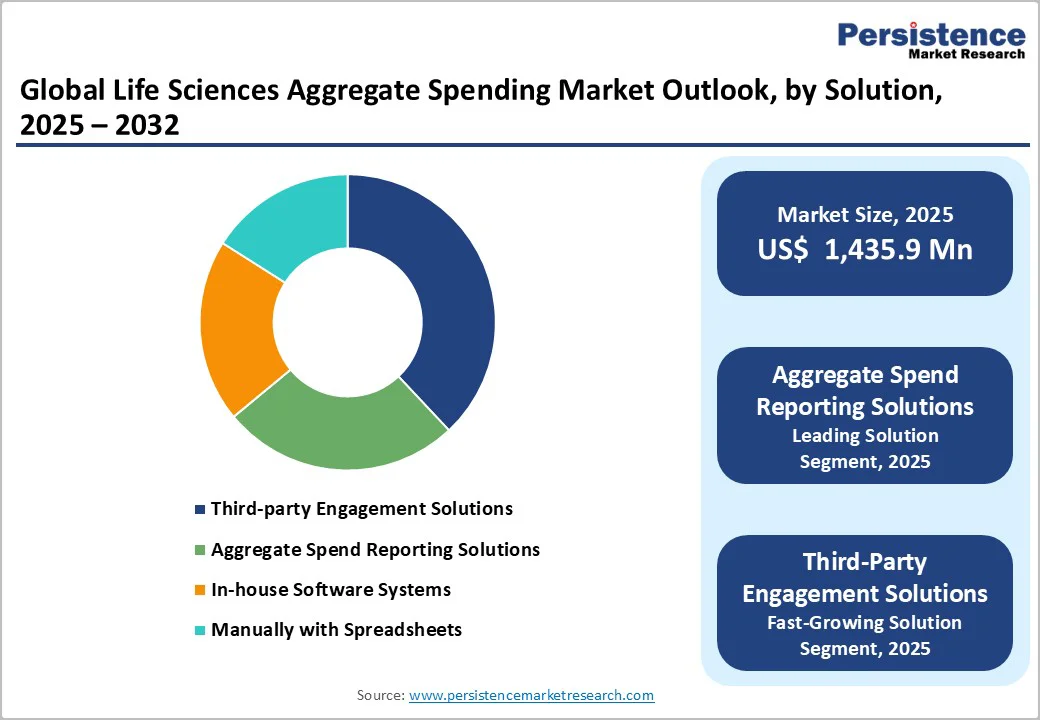
The Aggregate Spend Reporting Solutions segment leads the Life Sciences Aggregate Spending Market as organizations increasingly adopt advanced, automated platforms to meet complex global transparency regulations. These solutions provide seamless integration across multiple data sources, ensuring accuracy and audit readiness while minimizing manual workloads. Unlike in-house or spreadsheet-based systems, they offer real-time analytics, scalability, and multi-country compliance support. Continuous regulatory updates, AI-driven data validation, and customizable dashboards enhance efficiency and reduce compliance risks. As life sciences companies expand globally, the demand for centralized, cloud-enabled reporting systems positions this segment as the most trusted and widely implemented compliance solution.
The pharmaceutical companies segment holds the largest share in the Life Sciences Aggregate Spending Market due to its extensive financial interactions with healthcare professionals, hospitals, and research institutions. These include payments for consulting, sponsorships, clinical trials, and educational program activities strictly regulated under global transparency laws. To ensure compliance, pharma firms heavily invest in automated aggregate spend reporting systems that track and disclose transactions across jurisdictions. Their vast operational scale, higher compliance budgets, and early adoption of cloud-based and AI-enabled compliance tools further strengthen their dominance, making pharmaceutical companies the primary drivers of demand for advanced transparency and reporting solutions.
North America dominates the Life Sciences Aggregate Spending Market, driven by stringent regulatory frameworks and early digital adoption. The U.S. leads with strict transparency laws such as the Sunshine Act, mandating detailed disclosure of payments to healthcare professionals and organizations. Pharmaceutical and medical device companies in the region have rapidly embraced automated, cloud-based reporting platforms to ensure compliance and audit readiness. Continuous technological advancements, the presence of major compliance software providers, and high awareness of ethical healthcare practices further strengthen North America’s leadership. The region’s mature regulatory ecosystem and innovation-focused approach set global benchmarks for transparency and compliance management.
The Asia-Pacific region is emerging as a rapidly growing market in the Life Sciences Aggregate Spending landscape, driven by expanding pharmaceutical and biotechnology industries and evolving regulatory frameworks. Countries such as India, China, Japan, and South Korea are increasingly implementing transparency and ethical compliance norms similar to Western standards. Rising cross-border collaborations, growing clinical trial activity, and heightened government focus on healthcare accountability are fueling adoption of digital compliance and reporting tools. As multinational companies expand operations in Asia-Pacific, demand for cloud-based, multi-language, and cost-effective aggregate spend platforms is accelerating, positioning the region as a key future growth hub.

The global life sciences aggregate spending market is highly competitive, characterized by intense innovation and product differentiation. Leading players focus on high-quality, organic, and non-GMO flours, while premium and specialty variants, such as flavored or fortified life sciences, aggregate spending, and gain traction. Companies are investing in advanced processing techniques, attractive packaging, and expanded distribution through retail and e-commerce channels to enhance market presence. The growing demand for gluten-free, keto, and health-oriented products drives continuous product development.
The global life sciences aggregate spending market is projected to be valued at US$1,435.9 Mn in 2025.
Multinational operations require consistent data collection and reporting across diverse jurisdictions, boosting demand for automated and standardized compliance systems.
The global market is poised to witness a CAGR of 9.9% between 2025 and 2032.
Blockchain can ensure tamper-proof data trails and secure spend disclosures, building trust and simplifying audits across global operations.
Porzio Life Sciencess, LLC, MedPro Systems LLC, IQVIA Inc, Pharmagin, and others.
| Report Attribute | Details |
|---|---|
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis |
Value: US$ Mn |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
By Solution
By Deployment
By Business Type
By End-user
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author