ID: PMRREP17968| 198 Pages | 23 Jun 2025 | Format: PDF, Excel, PPT* | Healthcare

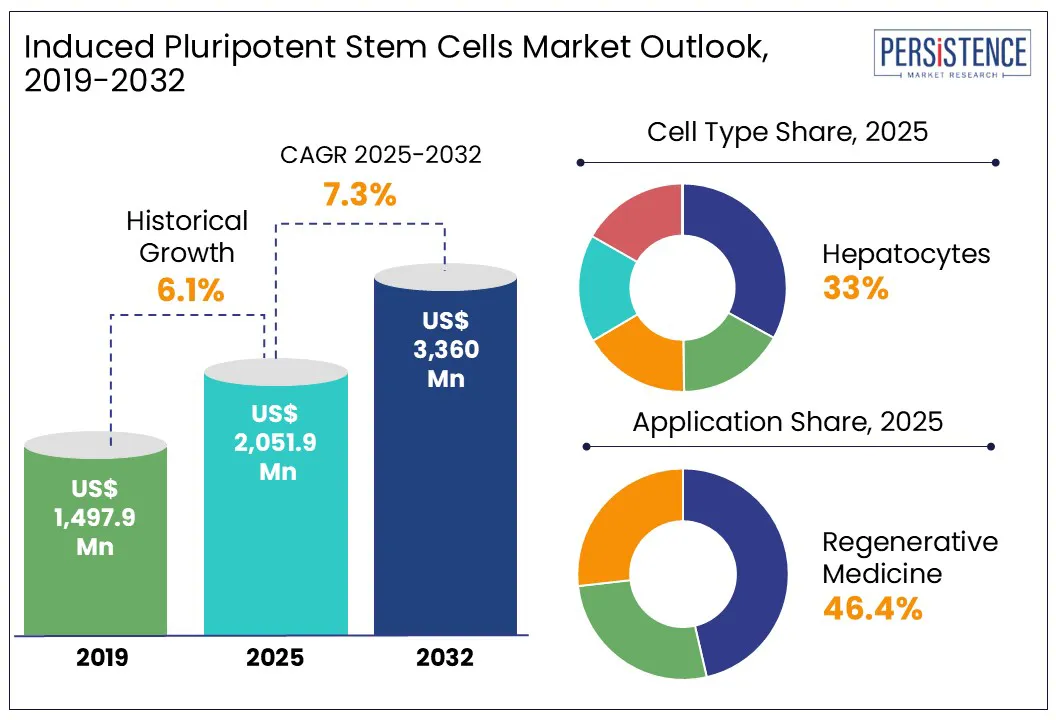
According to Persistence Market Research, the global induced pluripotent stem cells market size is likely to be valued at US$ 2,051.9 Mn in 2025 and is expected to reach US$ 3,360.0 Mn by 2032, growing at a CAGR of 7.3% during the forecast period from 2025 to 2032.
Growing investment in fundamental research, particularly by academic institutions and research organizations, has greatly enhanced the understanding of molecular pathways involved in disease development. In recent years, studies on induced pluripotent stem cells (iPSCs) have provided deeper insights into gene regulation, improving knowledge of disease mechanisms, diagnostic potential, and therapeutic strategies. iPSCs share a gene expression profile with embryonic stem cells (ESCs), retaining both pluripotency and self-renewal capabilities, making them invaluable for regenerative medicine and drug discovery.
A key trend in the industry is the increasing complexity of research demands, reflecting a growing understanding of biological pathways and the discovery of novel regulatory mechanisms. This evolution highlights the need for manufacturers of research tools and reagents to stay ahead of scientific advancements, ensuring they align with the rapidly evolving requirements of the life sciences sector.
Key Industry Highlights:
|
Market Attribute |
Key Insights |
|
Global Induced Pluripotent Stem Cells Market Size (2025E) |
US$ 2,051.9 Mn |
|
Projected Market Value (2032F) |
US$ 3,360.0 Mn |
|
North America Market Growth Rate (CAGR 2025 to 2032) |
7.3% |
|
Historical Market Growth Rate (CAGR 2019 to 2023) |
6.1% |
The increasing prevalence of chronic diseases is a major driver of growth in the Induced Pluripotent Stem Cells (iPSCs) market, as these cells offer promising applications in disease modeling, drug discovery, and regenerative medicine.
These trends are increasing the demand for iPSC-derived neuronal models, facilitating better disease understanding and the development of targeted therapies. As chronic diseases continue to rise, the iPSCs market is poised for sustained expansion.
iPSC-derived cell models are playing a crucial role in studying these diseases, developing personalized treatments, and advancing regenerative medicine. With chronic disease rates steadily climbing, the demand for iPSC applications in drug discovery and precision medicine is expected to drive significant market growth.
Increasingly complex requirements from researchers in the Life Science domain have led to the development of novel products that are expensive. Due to the uncertainties in the results expected from complex experiments, several users do not prefer to invest in such expensive products, especially for research groups with limited budgets and funding.
iPSC derivation is very costly, which is a limiting factor for most laboratories when multiple patient samples are reprogrammed. Custom research products and services are usually several-fold more expensive than products listed on catalogs of most of the major suppliers of research products. However, there is an increasing trend towards preference for premium products as they are of a higher quality than the standard reagents and products.
The global induced pluripotent stem cells (iPSCs) market presents significant future opportunities driven by advancements in regenerative medicine, precision therapies, and drug discovery. The increasing demand for personalized treatments is expected to boost iPSC-derived cell models for conditions such as Parkinson’s, Alzheimer’s, and cardiovascular diseases. With the global burden of neurodegenerative disorders projected to rise by over 50% by 2050, companies can leverage iPSCs to develop novel therapeutic strategies.
Additionally, the rising prevalence of conditions such as Alzheimer’s, Parkinson’s, and spinal cord injuries has fueled demand for iPSC-derived neuronal models. These models enable researchers to investigate disease mechanisms, test emerging therapies, and advance regenerative treatments.
Additionally, CRISPR gene editing and 3D bioprinting technologies are creating new avenues for tissue engineering and disease modeling. The expansion of automated cell culture systems will further enhance large-scale production, addressing cost and scalability challenges. Strategic collaborations between biotech firms and academic institutions are also fueling innovation. Emerging markets in Asia-Pacific and the Middle East provide untapped potential for expansion, with increasing government funding and research initiatives supporting iPSC-based therapies.
Among the various cell types, neurons held the largest share in the global induced pluripotent stem cell (iPSC) market. This is primarily attributed to the growing number of neurological disorders, such as Parkinson’s disease, Alzheimer’s, and multiple sclerosis, which demand advanced and effective treatment approaches. iPSCs offer promising potential in studying disease mechanisms, testing drug responses, and developing regenerative therapies tailored to these complex conditions.
Furthermore, the increasing use of iPSCs for patient-specific customization in treating non-curable diseases is supporting market growth. Their ability to differentiate into neurons makes them ideal for neurodegenerative disease research and therapy. Additionally, advancements in stem cell technology and an improved understanding of neural development are accelerating the adoption of neuron-derived iPSCs in research and clinical applications. The demand is expected to rise steadily as the need for personalized and regenerative solutions in neurology continues to grow globally.
In 2024, pharmaceutical and biotechnology companies accounted for the largest market share at 59.6%, driven by growing investments in developing stem cell-based therapies. These companies are actively working on new product pipelines to leverage the commercial potential of induced pluripotent stem cells (iPSCs). For example, in October 2022, Fate Therapeutics announced plans to showcase preclinical and clinical data from various iPSC platforms at the SITC meeting, underlining robust pipeline activity in the field. Meanwhile, the academic and research institutes segment is projected to witness the fastest growth during the forecast period. This is due to the increasing use of iPSCs in therapeutic and regenerative research.
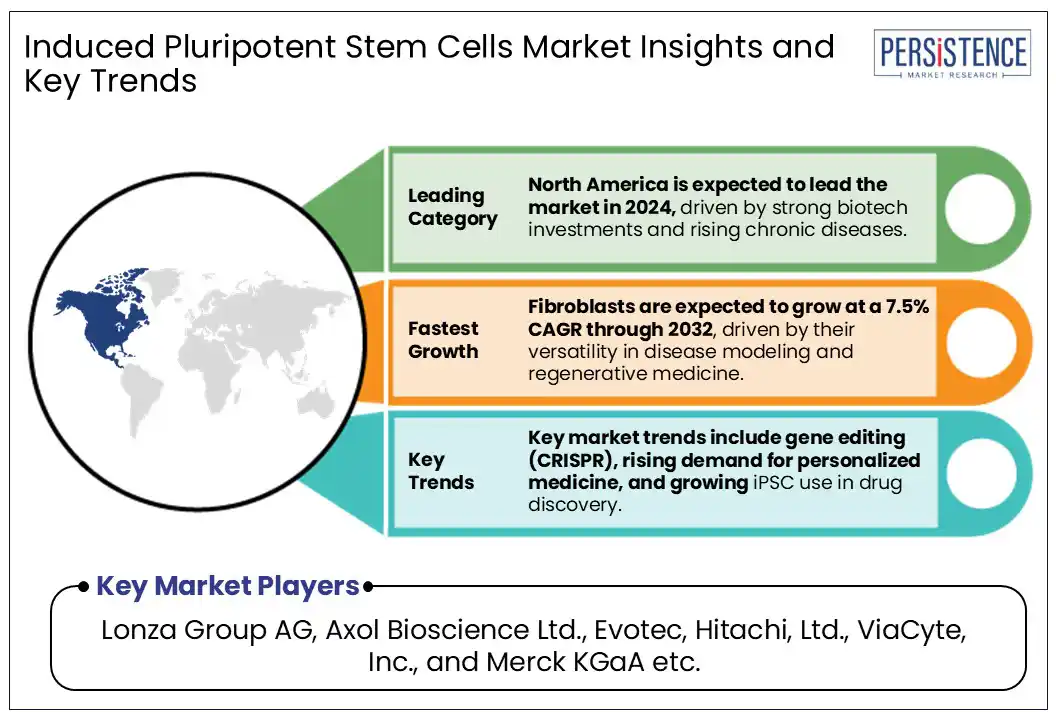
In 2024, North America is capturing a dominant 39.2% share of the global induced pluripotent stem cells (iPSCs) market, driven by the region’s increasing burden of chronic diseases and rising investments in regenerative medicine.
The U.S. Induced Pluripotent Stem Cells market, backed by robust funding from organizations such as the NIH, which allocated over US$2 billion for stem cell research in recent years. Additionally, the growing adoption of iPSC-derived therapies for neurodegenerative and cardiovascular diseases fuels industry expansion. With major biotech firms and academic institutions focusing on personalized treatments, North America remains at the forefront of stem cell advancements, fostering innovation and commercialization. This micromedicine procedure makes it possible to understand the progression of the disease at an individual patient level and also screen for optimal pharmacological drugs individually.
High-throughput analyses for drug toxicity that use human iPSC-CMs can also be conducted using micromedicine, under which iPSC-based medicine could be applied to cohorts of patients. In these clinical trials, iPSCs generated from patients could be differentiated into functional cells and are used to analyse the effectiveness of the drugs. The ongoing research in the region is expected to pave the way for future investments in the market.
The Europe induced pluripotent stem cells (iPSC) market is witnessing strong growth, supported by advanced research in biology, pharmaceuticals, and regenerative medicine. Key players and well-established R&D infrastructure across the region are major contributors. The UK market is expanding due to rising demand for cell therapies and a strong regulatory environment. In Germany, technological progress and growing interest in personalized therapies are driving increased investment in stem cell research. France is witnessing notable growth, fueled by a rise in clinical trials and expanding collaboration between domestic companies and research institutions, supported by a strengthening research and development ecosystem.
The Asia Pacific induced pluripotent stem cells (iPSC) market is experiencing significant growth, driven by factors such as a rising prevalence of chronic diseases, increased adoption of stem cell therapies, and advancements in biotechnology. Countries like China, Japan, and India are leading this growth due to substantial investments in research and development, favorable government policies, and a growing demand for personalized medicine. For instance, Japan has announced significant investments in establishing iPSC banks to support regenerative therapies.
Additionally, the region benefits from a large aging population, thereby increasing the demand for innovative treatments. Technological advancements, such as automated iPSC production processes, are also contributing to market expansion. However, challenges such as high costs associated with stem cell therapies and regulatory complexities may hinder growth. Overall, the Asia Pacific iPSC market is poised for robust expansion, offering lucrative opportunities for stakeholders in the coming years.
Despite the presence of several large-scale players as well as multiple regional companies in the global iPS cell market, there are very limited companies that have a complete focus on being a provider of life science reagents and products. Different manufacturers have different product offerings and specializations, with most large companies combining research products and reagents with equipment and in-vitro diagnostics tools. Having a sole focus on this market ensures better quality of products, customer loyalty, and an established distribution network that can increase the availability of the product across the globe.
The spending by companies on research and development about life sciences is expected to provide lucrative prospects for growth. Companies investing in providing tools and products to cater to the research requirements of several healthcare providers and research institutes can expect to see stable returns.
The spices are estimated to increase from US$ 2,051.9 Mn in 2025 to US$ 3,360.0 Mn by 2032.
Rising regenerative medicine demand, advancements in gene editing, increasing R&D funding, and expanding applications in drug discovery propel growth.
Lonza, Axol Bioscience Ltd., Evotec, Hitachi, Ltd., ViaCyte, Inc., and Merck KGaA are some of the leading industry players.
The market is projected to record a CAGR of 7.3% during the forecast period from 2025 to 2032.
Personalized regenerative medicine, leveraging iPSCs for patient-specific therapies, presents a major opportunity, driving advancements in disease modeling and drug discovery.
|
Attributes |
Details |
|
Current and Forecast Period |
2024 to 2032 |
|
Historical Data Available for |
2019 to 2023 |
|
Market Analysis |
US$ Million for Value |
|
Key Country Covered |
|
|
Key Market Segments Covered |
|
|
Key Companies Profiled |
|
|
Report Coverage |
|
|
Customization & Pricing |
Available upon request |
By Cell Type
By Application
By End User
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author