ID: PMRREP16768| 220 Pages | 5 Dec 2025 | Format: PDF, Excel, PPT* | Healthcare

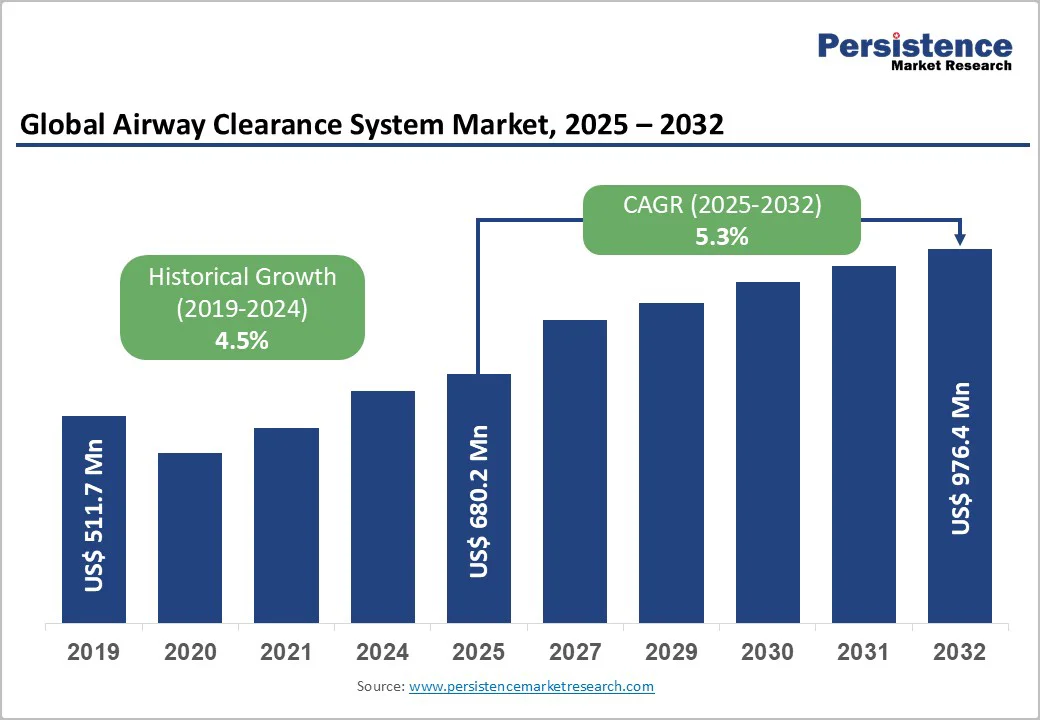
The global airway clearance system market size is valued at US$ 680.2 million in 2025 and projected to reach US$ 976.4 million by 2032.
The market is projected to record a CAGR of 5.3% during the forecast period from 2025 to 2032. The global market is growing steadily, driven by rising prevalence of chronic respiratory diseases, increasing home healthcare adoption, and technological advancements in devices such as HFCWO, PEP, and IPV systems.
Hospitals, pulmonary clinics, and home care settings are expanding usage. North America leads due to high awareness and reimbursement, while Asia Pacific is projected to achieve fastest-growth with rising patient population and healthcare access.
| Key Insights | Details |
|---|---|
| Global Airway Clearance System Market Size (2025E) | US$ 680.2 Mn |
| Market Value Forecast (2032F) | US$ 976.4 Mn |
| Projected Growth (CAGR 2025 to 2032) | 5.3% |
| Historical Market Growth (CAGR 2019 to 2024) | 4.5% |
Rising prevalence of chronic respiratory diseases (CRDs) is a powerful driver of the airway-clearance system market. Globally, approximately 468.3 million people were living with CRDs in 2021, according to the Global Burden of Disease Study. CRDs caused 4.4 million deaths and 108.5 million disability-adjusted life years (DALYs) in the same year.
Specifically, chronic obstructive pulmonary disease (COPD) affected 213.4 million individuals in 2021. These numbers indicate a huge and growing population in need of effective mucus-management therapies. As CRD patients frequently suffer from mucus accumulation, airway-clearance devices (like HFCWO vests, PEP, and IPV) are increasingly vital to reduce exacerbations, improve lung function, and enhance quality of life.
Lack of patient compliance is a major restraint in the airway-clearance system market, significantly affecting therapy outcomes and limiting market growth. Chronic respiratory diseases like cystic fibrosis (CF), bronchiectasis, and COPD require consistent use of devices such as high-frequency chest-wall oscillation (HFCWO) vests, positive expiratory pressure (PEP) devices, and intrapulmonary percussive ventilation (IPV) systems.
However, adherence is often low due to the time-consuming nature of treatments, complexity of device operation, and treatment fatigue. Studies indicate that only about 35% of CF patients maintain high adherence to HFCWO therapy, while nearly 28% of patients use it less than 35% of the prescribed time.
For non-CF bronchiectasis, consistent use of airway-clearance techniques is reported in only 41% of patients. Such low compliance reduces clinical efficacy, limits device adoption, and restrains overall market expansion.
The expansion of home healthcare solutions represents a major opportunity for the airway clearance system market. In the U.S., more than 3 million people receive home healthcare annually, with the majority of visits focused on managing chronic conditions, including respiratory diseases such as COPD, cystic fibrosis, and bronchiectasis.
Homecare allows patients to perform airway clearance therapy regularly using devices such as HFCWO vests, PEP systems, and intrapulmonary percussive ventilation units, reducing hospital visits and improving lung function. Studies show that home-based airway-clearance therapies improve patient adherence, reduce exacerbations, and enhance quality of life.
Moreover, telehealth integration and caregiver support further facilitate regular use and monitoring. As populations age and chronic respiratory conditions rise, the adoption of home healthcare solutions for airway clearance is expected to expand, offering patients convenience, autonomy, and more consistent therapy while driving long-term demand for airway-clearance devices.
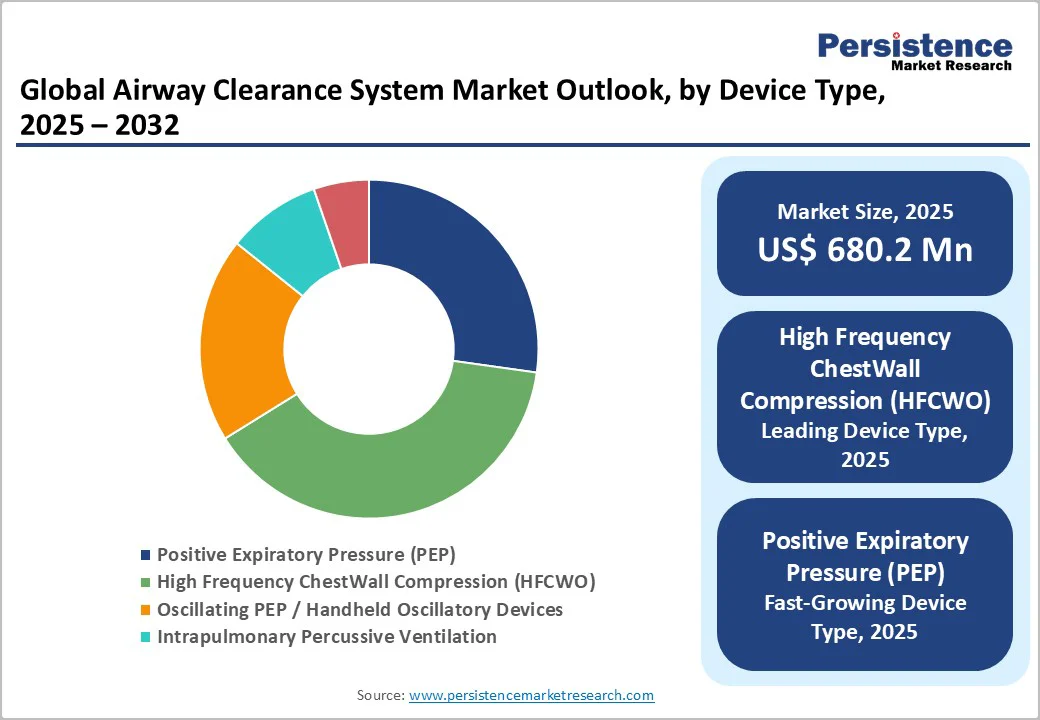
High Frequency ChestWall Compression (HFCWO) dominates the market with 39.0% share in 2025, because it consistently shows stronger clinical outcomes and wider patient suitability. Evidence from NIH-indexed clinical studies shows that HFCWO improves mucus clearance significantly-one meta-analysis of 13 randomized trials (756 patients) reported an increase of 6.18 mL in sputum expectoration and a reduction of 4.37 hospital days in acute COPD cases.
Peer-reviewed data in bronchiectasis also show airway flows up to 1.6 L/s and displacement volumes of 15-57 mL, leading to better FEV1 and FVC improvement than traditional physiotherapy. These government-backed and journal-verified outcomes demonstrate superior efficiency, non-invasiveness, and consistent performance, making HFCWO the most adopted device type globally.
Cystic Fibrosis (CF) dominates the airway clearance system market because airway-clearance therapy is an essential, lifelong requirement for every diagnosed patient. CF causes abnormally thick mucus that obstructs the lungs, making routine airway clearance mandatory. According to the Cystic Fibrosis Foundation Patient Registry, the U.S. had 38,804 CF patients in 2020, and over 70% required daily airway-clearance support.
Clinical guidelines from the Cystic Fibrosis Foundation recommend airway-clearance therapy for all CF patients, highlighting its central role in maintaining lung function. Cochrane-reviewed evidence also shows airway-clearance devices significantly improve mucus transport and short-term lung outcomes in CF. Because the therapy is universally indicated and used frequently, CF remains the largest and most stable application segment.
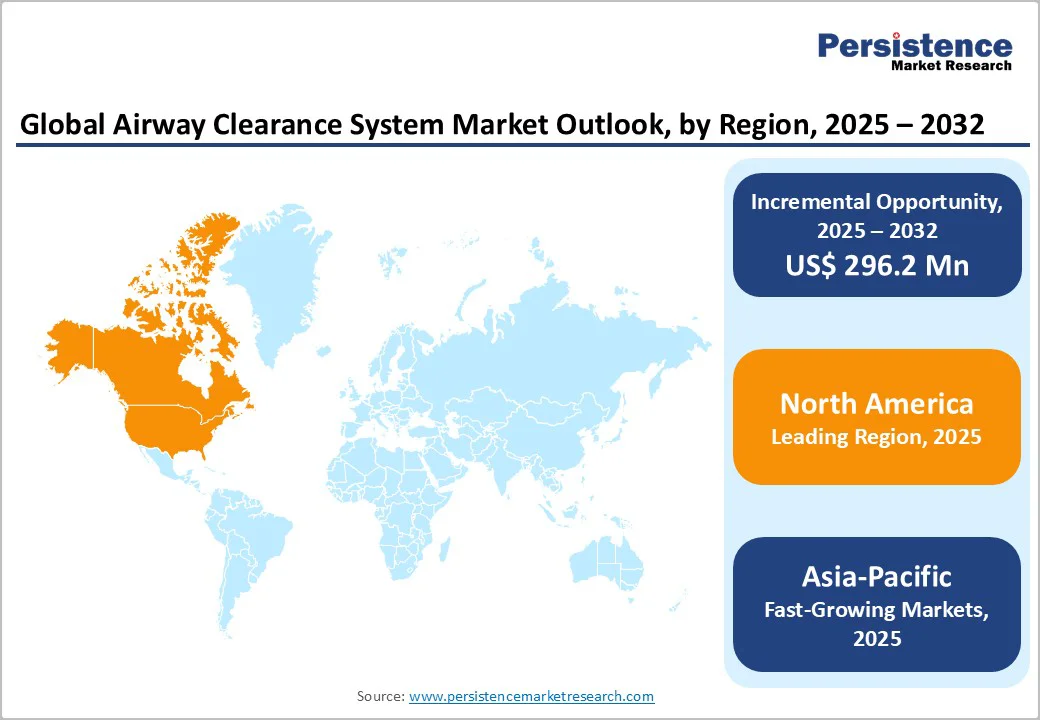
North America region dominates the global market with 40.5% share in 2025, due to its high respiratory disease burden, strong healthcare infrastructure, and widespread use of advanced airway-clearance technologies. The U.S. has one of the world’s largest cystic fibrosis (CF) populations, with 38,804 patients recorded in 2020 according to the CF Foundation Patient Registry.
The region also has a high COPD burden, 16 million diagnosed and many more undiagnosed, as reported by the CDC driving consistent demand for airway-clearance devices. Furthermore, the U.S. reports a CF incidence of 1 in 3,500 births, ensuring continuous need for lifelong airway management. Well-established reimbursement systems, specialized CF care centers, and rapid adoption of FDA-cleared devices further strengthen North America’s market leadership.
Asia Pacific is expected to experience fastest-growth in the airway clearance system market due to its rapidly rising burden of chronic respiratory diseases, high pollution levels, and improving healthcare access. Epidemiological studies estimate a 6.3% COPD prevalence across major Asia Pacific countries, representing over 56 million adults.
The Global Burden of Disease analysis reports 52.1 million COPD cases in East Asia and 43.8 million in South Asia, indicating one of the world’s highest disease loads. WHO data also show 1.56 million deaths from chronic respiratory diseases in Southeast Asia, driven by PM2.5 pollution and biomass fuel exposure.
As hospitals modernize and home-care awareness increases, demand for airway-clearance devices accelerates, making Asia Pacific the fastest-growing regional market.
Europe is an important region in the airway clearance system market due to its large respiratory disease burden, organized patient registries, and strong healthcare infrastructure. The WHO/European Respiratory Society reports that over 80 million people in the WHO European Region are living with chronic respiratory diseases, including COPD, asthma, and bronchiectasis. COPD alone affects 36 million Europeans, creating substantial demand for airway-clearance support.
Additionally, the European Cystic Fibrosis Society Patient Registry records 54,000+ CF patients across more than 40 countries, all requiring lifelong airway-clearance therapy. Europe’s universal healthcare systems, reimbursement structures, and wide availability of specialized respiratory clinics further support high adoption of airway-clearance devices, making it one of the most significant global market regions.
The global airway clearance system market is expanding steadily, driven by rising COPD, asthma, and cystic fibrosis cases, along with growing preference for noninvasive respiratory therapies. Hospitals and home-care settings increasingly adopt HFCWO, PEP, and oscillatory devices for better clinical outcomes. North America leads due to strong infrastructure, while Asia Pacific grows fastest owing to pollution burden, healthcare expansion, and improving affordability.
The global airway clearance system market is valued at US$ 680.2 Mn in 2025.
Rising respiratory diseases, aging populations, pollution exposure, and growing adoption of noninvasive airway-clearance devices collectively drive the global market.
The global airway clearance system market is poised to witness a CAGR of 5.3% between 2025 and 2032.
Expanding home-based respiratory care, device digitalization, AI-enabled monitoring, rising Asia Pacific demand, chronic disease screening programs, and improved reimbursement for advanced airway-clearance systems.
Baxter, Electromed, Inc, Koninklijke Philips N.V., General Physiotherapy II LLC, Med Systems, Inc., and VYAIRE.
| Report Attribute | Details |
|---|---|
| Historical Data/Actuals | 2019 - 2024 |
| Forecast Period | 2025 - 2032 |
| Market Analysis | Value: US$ Mn; Volume: Units |
| Geographical Coverag |
|
| Segmental Coverage |
|
| Competitive Analysis |
|
| Report Highlights |
|
By Device Type
By Application
By End-user
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author