ID: PMRREP35425| 197 Pages | 19 Jun 2025 | Format: PDF, Excel, PPT* | Healthcare

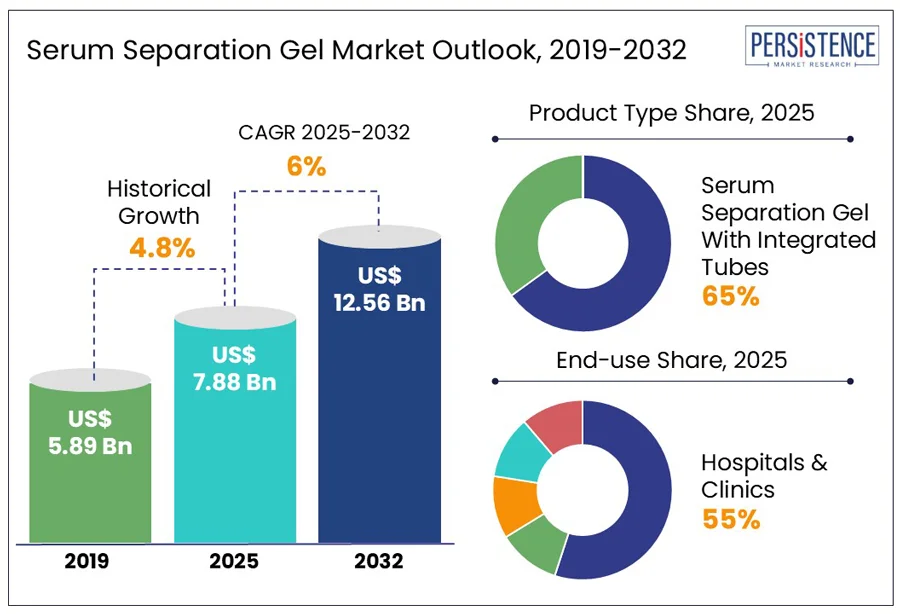
The global serum separation gel market size is projected to rise from US$ 7.88 bn in 2025 to US$ 12.56 bn by 2032. The market is anticipated to register a CAGR of 6% during the forecast period from 2025 to 2032. According to the Persistence Market Research report, increasing disease burden across the globe, rapid advancement of point-of-care diagnostic testing devices, rising awareness of preventive healthcare and early treatment, and growing disposable incomes that favor testing have led to market expansion over the forecast period.
Serum separation gel is a high-molecular-weight compound used in disposable vacuum blood collection tubes or injection blood collection tubes. Blood collection and testing are critical to medical research, diagnosis, monitoring, and treatment, as they provide information about patients to medical professionals. The blood chemistry, electrolyte levels, and blood cells are indicators of diseases or the absence of diseases. Serum-separating gel and additives for blood collection help in separating blood components, maintaining blood clotting, involving less blood for analysis, and preserving the sample stability without any contamination. It helps medical staff quickly procure high-quality serum samples.
Key Industry Highlights
|
Global Market Attribute |
Key Insights |
|
Serum Separation Gel Market Size (2025E) |
US$ 7.88 Bn |
|
Market Value Forecast (2032F) |
US$ 12.56 Bn |
|
Projected Growth (CAGR 2025 to 2032) |
6.00% |
|
Historical Market Growth (CAGR 2019 to 2024) |
4.80% |
Rising incidences of infectious diseases, growing disposable incomes, and awareness about preventive healthcare are amping up the demand for diagnostic services. According to the Centers for Disease Control and Prevention (CDC), nearly 6 in 10 Americans suffer from at least one chronic disease, boosting the need for regular diagnostic procedures involving blood tests. For example, Diabetes Mellitus requires serum for tests such as glucose, lipid profiles, and renal function to monitor disease management. HIV/AIDS testing, Hepatitis B and C, and COVID-19 diagnostics also use serum separator tubes for antibody and cytokine tests. As healthcare systems worldwide continue to highlight accurate and efficient diagnostic processes, the role of serum separation gels becomes more critical in guaranteeing reliable test results.
The availability of home collection services by laboratories has also increased the rate of blood testing. Increasing diagnosis of hereditary bleeding disorders, such as hemophilia A (factor VIII deficiency), hemophilia B (factor IX deficiency), and von Willebrand disease, also requires blood tests and blood transfusions, which has surged the requirement for serum separation gels. These gels are extensively used to facilitate and simplify the separation of serum from blood samples during diagnostic testing, to analyze various biomarkers, enzymes, hormones, and other components. Remote and underdeveloped areas have seen a rise in point-of-care diagnostic testing, thereby offering significant opportunities for serum separation gels to be employed.
Serum separation gels can malfunction during practical use, leading to bubble formation and separation gel drawing. Air gets trapped in the gel during its injection into the bottom of the blood collection tube manually or by machine, causing bubbles. These air bubbles can interfere with the gel’s ability to form an effective barrier between serum and cells, jeopardizing sample quality and test accuracy. Separation gel drawing denotes the unintended movement or displacement of the gel barrier after centrifugation, causing incomplete separation of the serum from cellular components. Improper tube handling, centrifugation parameters, or temperature fluctuations can lead to these issues, highlighting the importance of strict quality control during manufacturing and careful handling in the lab to ensure reliable and accurate diagnostic results.
Emerging technologies such as solid-phase extraction (SPE) and microfluidic devices are taking the place of serum separation gels. Microfluidic devices are very useful at the point-of-care as they can separate serum or plasma quicker than traditional centrifugation, enabling rapid diagnostics with fewer errors. Both SPE and microfluidics require smaller blood volumes, which smooths the process for pediatric patients. The portability of microfluidic devices also enables easy testing in remote or resource-constrained environments. Plasma Separator Tubes (PSTs) are also becoming popular for tests requiring plasma instead of serum, such as coagulation studies and certain molecular diagnostics, as they offer faster processing.
Ongoing technological advancements have led to the development of modern gels such as thermo-stable gel formulations, nanocomposite-based gels (faster and cleaner separation), and polymeric gels (high chemical inertness). The integration of rapid-setting clot activators and multi-tube compatibility has increased their versatility across various diagnostic platforms. These innovative gels with longer shelf life, greater sample purity, and broader compatibility with a range of assays make them essential in both laboratory and point-of-care settings. The quality of the blood sample enables healthcare providers to make informed decisions and reduce errors and delays.
The gel is now integrated into automated systems, reducing manual handling and enhancing the overall lab productivity. Recent developments focus on improving gel layer consistency, stability, and tube durability to minimize contamination and ensure optimal serum or plasma separation. Moreover, enhanced tube designs and the use of barcoding and electronic data systems improve traceability and reduce human errors. As these technologies continue to evolve, gel separators are expected to remain essential in providing efficient healthcare services.
The serum separation gel with integrated tubes segment is anticipated to dominate the serum separation gel market, capturing around 65% of revenue over the forecast period. These serum separator tubes (SSTs), are specialized blood collection tubes that contain a gel to separate serum from blood cells after centrifugation. The gel enhances sample purity and makes handling easier, forming a stable barrier between the clot and the serum. These tubes optimize time utilization, reduce contamination, enhance sample stability, and support automation in diagnostic processes.
A study conducted to compare two sample preparation methods for MALDI-ToF MS identification of pathogens in 200 positive blood cultures found that the SST method outperformed differential centrifugation, particularly in identifying Gram-positive bacteria (83.3% vs. 65.3%), and was significantly faster. Becton Dickinson, Greiner Bio-One, Terumo Corporation, Sarsted, and Medline Industries are the key manufacturers.
The serum separation gel without integrated tubes segment involves the manual addition of gel into blood collection containers, primarily in research or specialized laboratory settings where customized sample preparation is required. This approach offers flexibility in gel volume and composition, making it suitable for experimental protocols or non-standard tube sizes.
The hospitals & clinics segment accounted for the largest share of the serum separation gel market revenue, accounting for 55% over the forecast period. There is a high requirement for serum separation gel in hospitals due to the high volume of surgeries and diagnostic procedures conducted. New hospitals built in developing nations also drive market growth. According to CEIC, in 2024, there were 39,000 hospitals in China, making it one of the fastest-growing industries in the country. The need for greater efficiency, accuracy, and reliability in diagnostic workflows drives the adoption of serum separation gels in hospitals and clinics. With high patient volumes and increased pressure on laboratories to deliver faster results, serum separation gels help reduce the need for manual sample processing, accelerating the turnaround times.
The blood bank segment is anticipated to be the fastest-growing segment. According to the WHO, about 118.54 million blood donations are collected worldwide. 40% of these are collected in high-income countries, home to 16% of the world’s population. About 13,300 blood centers in 169 countries report collecting a total of 106 million donations. Serum separation gel tubes ensure rapid and clean separation of serum from blood cells, which is essential for these tests. The FDA’s Center for Biologics Evaluation and Research (CBER) regulates the collection, testing, and safety of blood and blood products by setting quality standards. The FDA aims to reduce risks to the lowest by screening blood for multiple infectious agents, in turn driving market growth.
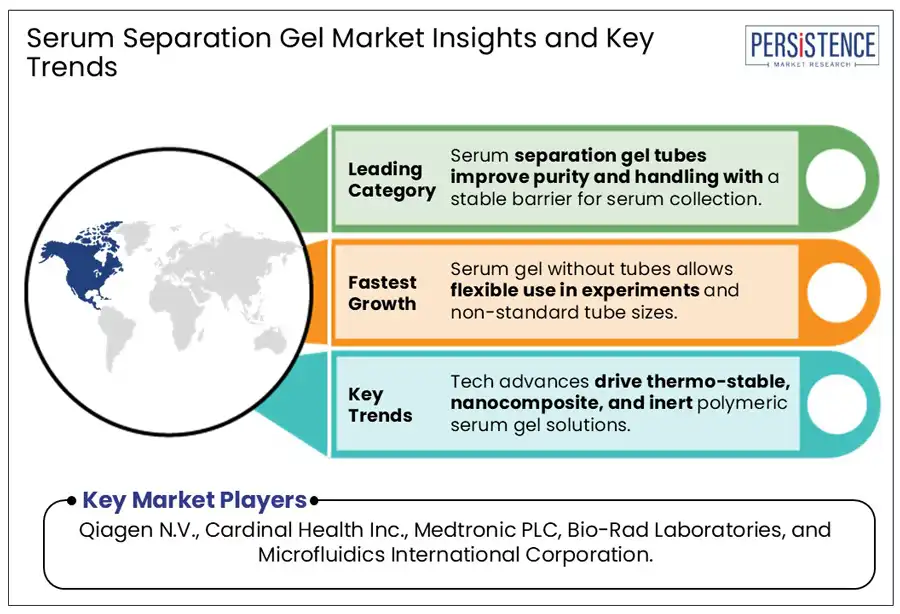
North America dominates the serum separation gel market, holding over 50% share from 2025 to 2032, driven by sophisticated medical infrastructure and a surge in clinical testing. The dominance is further supported by a sharp rise in hematological and infectious diseases, with a significant portion of cases among seniors due to comorbidities. The growing focus on early intervention, improved co-pay systems, and the strong footing of modern blood banks promote market uptake. Rising healthcare expenditures, expanding diagnostic applications, and a preference for digital health services continue to fuel the market demand across hospitals and clinics in the region.
The U.S. holds a sizeable market share within North America, largely due to the high incidence of bloodstream infections and chronic conditions, particularly among the elderly. According to the CDC data, candidemia ranks as the fourth most common healthcare-associated bloodstream infection, affecting approximately 25,000 people annually. The country has also witnessed robust blood donation activity, with over 6.8 million donors contributing about 13.6 million units each year.
The market in Asia Pacific is growing rapidly, driven by advancements in healthcare infrastructure, rising chronic disease prevalence, and increased diagnostic testing demand. The market includes serum separation gels with integrated tubes and standalone gels; however, those with integrated tubes dominate due to convenience. Key end-users are blood banks, hospitals, pharmaceutical firms, and research labs. India leads regional growth, supported by rising healthcare investments and expanding medical facilities. Japan also contributes to high growth, reflecting the region’s diverse healthcare landscape and expanding diagnostic capabilities.
China is anticipated to dominate over the forecast period. This leadership stems from China’s extensive healthcare infrastructure, rapid blood bank expansion, and rising diagnostic testing demand. Japanese company SEKISUI Chemical is a well-known foreign manufacturer of serum separation gel operating in China. Among domestic producers, Hubei New Desheng Material Technology Company has earned a strong reputation, offering products comparable in quality to international brands. Another notable local manufacturer is Shanghai Tenghu Biological Technology Company.
The European market is witnessing steady growth due to an aging population, increased demand for diagnostic testing, and advancements in healthcare infrastructure. Additionally, there is a growing trend toward gel-free separation methods offering faster processing. Stringent regulatory frameworks under the EU Medical Device Regulation (MDR), ensuring safety and quality compliance also favor the market.
The increasing prevalence of diabetes and cardiovascular conditions is further propelling the market, with healthcare providers emphasizing early diagnosis and preventive care, contributing to the expanding use of serum separation gels across hospitals, diagnostic labs, and research institutions. Key players operating in Europe include Greiner Bio-One, Sarstedt AG & Co., and Thermo Fisher Scientific.
Germany dominates in Europe due to its advanced healthcare system and strong medical device manufacturing sector. The country benefits from high healthcare expenditure, a large pool of elderly population, and a robust diagnostic testing infrastructure. Germany’s strict regulatory environment and emphasis on R&D drive continuous improvements, making it a key market for serum separation technologies. Major German companies such as Sarstedt AG & Co. and Greiner Bio-One lead innovation in serum separation gel products, focusing on high-quality, reliable solutions for clinical diagnostics.
The global serum separation gel market is highly competitive and fragmented, with numerous established global and regional players offering a wide range of products and vying for higher market share. Key players are focusing on developing innovative product solutions, such as serum fast separator tubes, to distinguish themselves. The market is experiencing growth due to the high demand for diagnostic testing and rising healthcare expenditure.
The global market is projected to be valued at US$ 7.88 bn in 2025.
The serum separation gel market is fueled by the increasing disease burden across the globe and rapid advancement of point-of-care diagnostic testing devices.
The market is poised to witness a CAGR of 6% from 2025 to 2032.
Ongoing technological advancements have led to the development of modern gels such as thermo-stable gel formulations, nanocomposite-based gels that enable faster and cleaner separation, and polymeric gels with high chemical inertness.
Major players in the Serum Separation Gel industry are Qiagen N.V., Cardinal Health Inc., Medtronic PLC, Bio-Rad Laboratories, and Microfluidics International Corporation.
|
Report Attribute |
Details |
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis |
Value: US$ Bn |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
|
Customization and Pricing |
Available upon request |
By Product Type
By End-use
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author