ID: PMRREP15373| 198 Pages | 17 Nov 2025 | Format: PDF, Excel, PPT* | Healthcare

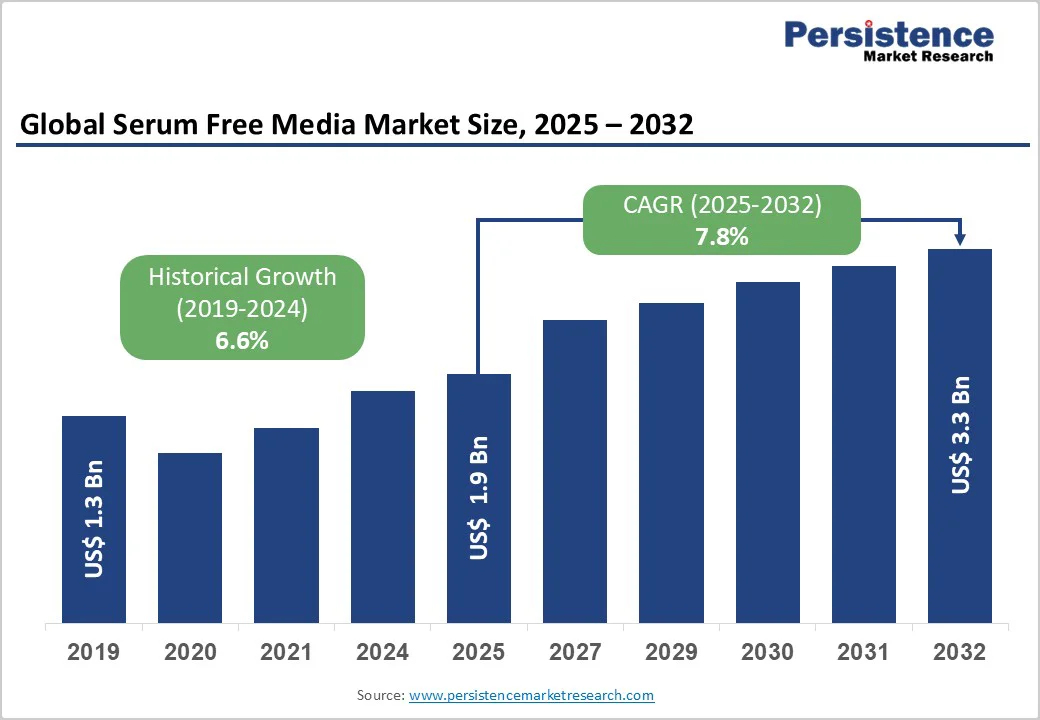
The global serum-free media market is expected to reach US$1.9 billion in 2025 and US$3.3 billion at a CAGR of 7.8% during the forecast period from 2025 to 2032. Serum-free media (SFM) supports the growth of a specific cell type with a particular function, such as providing growth factors and hormones to support cell function and promote development in cell culture under predetermined conditions.
The growth of research and development is increasing owing to increased research and development activities and the extensive use of animal-component-free media, such as those lacking clotting proteins, interfering components, glucose, insulin, transferrin, and selenium that affect cell growth. Cell culture has broad applications in disease diagnosis, underscoring its importance in healthcare.
It ascertains the safe and effective application of upgraded cell culture and cell therapy methods. Other uses of SFM cell cultures are cancer research, cell biology, metagenomics, molecular biology applications, and plant research. Various clinical organizations and biopharmaceutical companies are working together to identify the therapeutic applications of pharmaceutical drugs across several disease conditions to facilitate systematic diagnosis, appropriate dosing, and treatment selection, and treatment monitoring.
| Key Insights | Details |
|---|---|
|
Serum Free Media Market Size (2025E) |
US$1.3 Bn |
|
Market Value Forecast (2032F) |
US$1.9 Bn |
|
Projected Growth (CAGR 2025 to 2032) |
7.8% |
|
Historical Market Growth (CAGR 2019 to 2024) |
6.6% |
Cell-specific serum-free media (SFM) offer homogeneity, enhancing cell growth and minimizing inconsistencies in cell culture. This uniformity contributes to improved productivity in biopharmaceutical research, development, and manufacturing. Each component in SFM is precisely defined, enabling consistent cell culture performance and reducing contamination risks from fungi, bacteria, and other pathogens. Compared to serum-based or undefined media, SFM simplifies purification processes by providing a known composition and lower protein concentration, making it easier to control the cellular environment and minimize downstream contamination.
The reduced protein content also reduces interference between serum and recombinant proteins, thereby improving the accuracy of recombinant protein studies. Academic and industrial researchers increasingly prefer SFM for innovative biopharmaceutical research and the development of novel disease treatments. As biopharmaceuticals gain prominence for their effectiveness in treating chronic diseases, their production volume continues to grow rapidly. Constant advancements in production technologies and molecular innovations further expand market potential.
Additionally, the COVID-19 pandemic positively influenced the SFM market, as leading biopharmaceutical companies intensified in vitro research efforts to develop vaccines and antiviral therapies, highlighting the critical role of serum-free media in modern biomedical research.
Serum provides natural growth mediators essential for cell culture, but in serum-free media (SFM), these are replaced with defined artificial growth factors. Because the requirements for growth mediators differ across cell types, some cells may exhibit slower growth rates. To qualify as a defined medium, every SFM component, along with its concentration and function, must be precisely known. However, not all cell types respond uniformly to a specific SFM formulation. Developing cell-specific SFM involves extensive screening and optimization at each stage, making the process complex, costly, and time-consuming. This significantly increases research and production expenses.
Additionally, creating SFM tailored to particular cell types further extends development timelines. The diverse growth factor needs of various cells influence overall demand for SFM. Moreover, stringent regulatory evaluations and the requirement to assess every component delay market availability. These challenges, coupled with regional disparities in production and supply, continue to restrict the broader adoption and expansion of serum-free media globally.
The Serum-Free Media (SFM) market is witnessing strong growth opportunities driven by the rising global demand for vaccines and monoclonal antibodies. The growing focus on advanced cell-based research and the expansion of R&D capabilities are encouraging greater adoption of serum-free technologies. Rapid advancements in genomic and proteomic profiling, genetic engineering, and recombinant DNA techniques have further enhanced the need for well-defined and contamination-free cell culture media.
Biopharmaceutical companies are increasingly partnering with academic institutions to leverage expertise, research facilities, and innovative resources for improved cell media development and large-scale manufacturing. Additionally, the surge in contract research organizations (CROs) and biopharmaceutical manufacturing units is driving demand for reliable, high-quality serum-free formulations. Key industry players are responding by broadening their product portfolios with specialized, cell-specific media that ensure reproducibility and minimal genetic variability. These combined factors position SFM as a vital enabler of efficient, safe, and scalable biologics production, creating substantial opportunities for market expansion in the coming years.
CHO cell culture media holds a commanding position in the serum-free media market, commanding approximately 24% market share in 2025, making it the leading media type. This dominance reflects CHO cells' extensive applications in biopharmaceutical manufacturing, particularly for monoclonal antibody production and recombinant therapeutic protein expression. CHO cell systems have become the industry standard for producing biologics, with superior productivity rates, established regulatory pathways, and proven scalability from research to commercial-scale manufacturing. The stability, reliability, and well-characterized biology of CHO cells, combined with continuous optimization of specialized serum-free formulations, have solidified their market leadership. Additionally, the high success rate of CHO cell-derived therapeutics in clinical applications and the significant capital investment by major manufacturers in CHO cell platform development reinforce this segment's dominance in the global serum-free media market.
Biopharmaceutical companies represent the dominant end-user segment, capturing approximately 70% of the serum free media market share in 2025, reflecting their critical role in advanced therapeutic development and manufacturing. These organizations maintain substantial resources for implementing sophisticated cell culture systems, investing heavily in serum-free media to ensure consistent production of high-value therapeutics, including monoclonal antibodies, recombinant proteins, and cell therapies.
The segment's dominance reflects regulatory requirements, quality standards, and cost-justification through high-value product portfolios. Clinical Research Organizations and Academic Research Centers, while representing smaller market segments, demonstrate increasing adoption of serum-free systems for clinical trial support and translational research applications, though their adoption rates remain constrained by budget limitations and infrastructure constraints compared to large pharmaceutical enterprises.

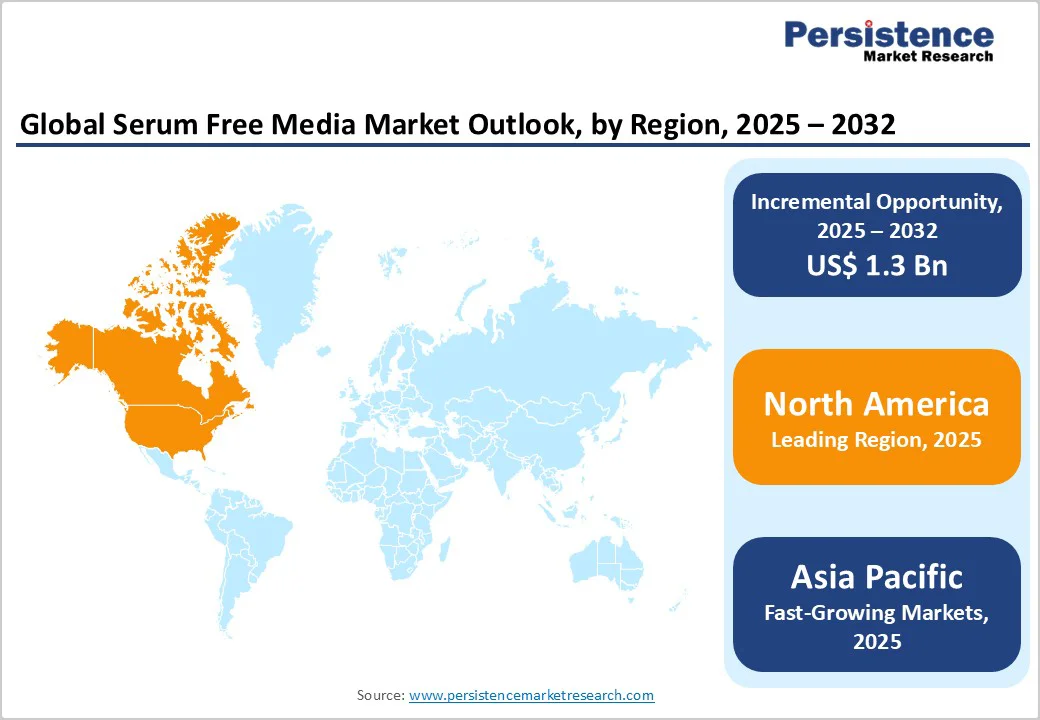
North America maintains leadership in the global serum free media market, driven by a mature and sophisticated biotechnology ecosystem centered in the U.S. The region's dominance reflects established biopharmaceutical sectors, substantial government research funding from the National Institutes of Health (NIH) and BARDA, and advanced regulatory frameworks supporting innovation. The U.S. alone accounts for approximately 35% of global biopharmaceutical production, with serum-free media playing an indispensable role in manufacturing processes.
Leading biopharmaceutical companies including Thermo Fisher Scientific, Merck, and Lonza, maintain significant research and manufacturing operations throughout North America, supporting continuous innovation in media formulations. Regulatory support from the FDA for animal-origin-free manufacturing, combined with high disease prevalence driving demand for innovative therapeutics, ensures sustained market expansion. Investment initiatives focused on cell-based therapies and vaccine development infrastructure further reinforce North America's position as the leading regional market for serum-free media solutions.
Asia Pacific emerges as the fastest-growing regional market for serum-free media, driven by accelerating biopharmaceutical sector expansion, supportive government policies, and increasing investments in biotechnology infrastructure. In China, the prevalence of infectious and chronic diseases is rising despite all precautions and personal hygiene practices. The prevalence of infectious diseases has led to improvements in research and development using serum-free media technology, which is anticipated to drive the total serum-free media market.
Government support for the production of vaccines based on cell cultures, amiable regulatory requirements, low manufacturing costs, increasing research and development capabilities with the aid of key manufacturers, increased focus on collaboration with China, and rising stem cell and cell culture therapies, will all contribute to the market expansion in China.
Indian pharmaceutical companies are expanding their biologics portfolios through government support programs, including the DBT initiative. Japan's regenerative medicine sector demonstrates robust growth following the enactment of the Act on the Safety of Regenerative Medicine, with demand for specialized stem cell culture media. Regional manufacturing advantages, including skilled workforce availability, cost-effective production capacity, and favorable regulatory environments, attract global biopharmaceutical investments, positioning the Asia Pacific as a strategic growth region for serum-free media expansion.
The global serum-free media market is highly competitive, with major players focusing on innovation and capacity expansion. Leading companies such as Thermo Fisher Scientific, Merck KGaA, Lonza Group, and Corning Incorporated are investing in advanced cell culture technologies and developing chemically defined, animal-component-free formulations.
Strategic collaborations, mergers, and acquisitions are strengthening their global presence and product portfolios. Regional manufacturers are also entering the market with cost-effective and customized solutions to meet growing biopharmaceutical and cell therapy demands. Continuous R&D efforts, regulatory compliance, and GMP-certified production capabilities remain key differentiators in this evolving and quality-driven market landscape.
The global serum free media market is projected to be valued at US$ 1.9 Bn in 2025.
Rising prevalence of chronic and infectious diseases, and growing investments in research, are the key factors propelling growth within the global market.
The global market is poised to witness a CAGR of 7.8% between 2025 and 2032.
Rising biologics, cell therapy, and cultured meat demand create opportunities for defined, GMP-grade serum-free media.
Companies such Thermo Fisher Scientific Inc., Merck KGaA, GE Healthcare, Lonza, Corning Incorporated, Irvine Scientific are some of the major players operating in the global Serum Free Media Market.
| Report Attribute | Details |
|---|---|
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis |
Value: US$ Bn and Volume (if Available) |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
By Media
By Application
By End User
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author