ID: PMRREP13957| 200 Pages | 15 Oct 2025 | Format: PDF, Excel, PPT* | Healthcare

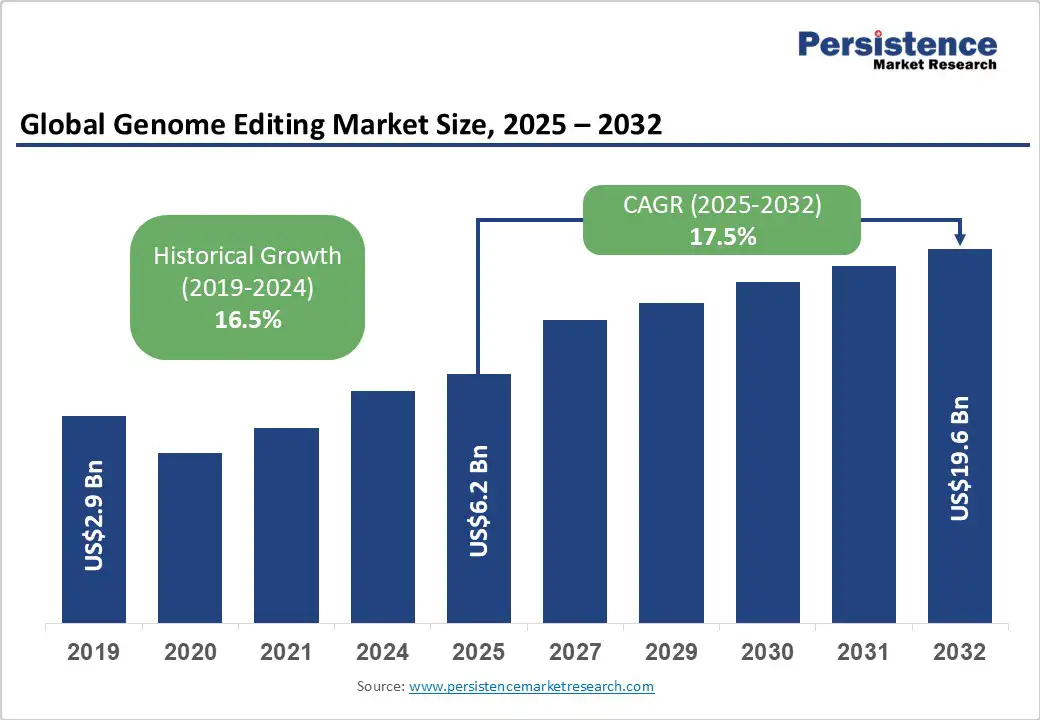
The global genome editing market size is likely to be valued at US$6.2 billion in 2025. It is estimated to reach US$19.6 billion by 2032, growing at a CAGR of 17.5% during the forecast period 2025 - 2032, driven by the rising prevalence of genetic disorders and cancer, breakthroughs in genome editing techniques such as CRISPR/Cas9 and base and prime editing, and the growing adoption of these technologies in the pharmaceutical and agricultural biotechnology sectors.
| Key Insights | Details |
|---|---|
|
Genome Editing Market Size (2025E) |
US$6.2 Bn |
|
Market Value Forecast (2032F) |
US$19.6 Bn |
|
Projected Growth (CAGR 2025 to 2032) |
17.5% |
|
Historical Market Growth (CAGR 2019 to 2024) |
16.5% |
Advancements in gene editing technologies, especially the widespread adoption of CRISPR/Cas9, base editing, and prime editing, significantly enhance precision and reduce off-target effects. For instance, prime editing, which enables precise DNA insertions without double-stranded breaks, is expected to accelerate therapeutic development pipelines. The increasing number of clinical trials utilizing these tools is projected to drive market growth, with contributions from evolving delivery systems, such as lipid nanoparticles, enhancing safety and efficiency. Global investment in genomics R&D, exemplified by the US$814 Million funding by the U.S. National Institutes of Health (NIH) for gene editing projects, is bolstering these technological leaps, enabling scalable therapeutic applications and cost-effective treatments.
The escalating burden of cancer, genetic, and rare diseases globally is intensifying demand for genome editing solutions. The 2022 report of the International Agency for Research on Cancer (IARC) revealed that cancer caused 9.7 million deaths in 2022 and 20 million new cancer cases were reported worldwide, escalating the necessity for innovative therapeutic strategies, including gene therapies. Genome editing technology supports the development of personalized medicine targeting the underlying genetic causes, thus expanding clinical applications. This trend, coupled with demographic shifts such as aging populations in developed nations, is expected to sustain long-term demand for these technologies across the pharmaceutical and healthcare sectors.
Government agencies worldwide are actively supporting gene editing research and clinical translation. In the U.S., programs such as the Somatic Cell Genome Editing (SCGE) initiative have received over US$140 Million to accelerate clinical applications. Regulatory bodies are gradually establishing frameworks facilitating gene editing technology adoption, illustrated by the U.K.’s elimination of risk assessments for gene-edited plant trials. Such policy developments reduce market entry barriers and accelerate product commercialization, fostering innovation and expanding biotechnological applications in both therapeutic and agricultural domains.
Despite technological progress, off-target gene editing remains a significant challenge, causing unintended DNA modifications that may lead to hazardous outcomes. Single-guide RNA mistargeting risks hinder broader clinical adoption, necessitating stringent safety evaluations and regulatory scrutiny. Viral vectors used for gene delivery, such as adeno-associated viruses, require extensive safety protocols to avoid genome integration risks, adding complexity and cost to the development process. Ongoing safety concerns slow the transition of some gene editing applications from research to commercial therapies.
The complexity and high cost of gene editing workflows, especially for ex vivo therapies, pose barriers to scale-up. Limited manufacturing slots at specialized contract development and manufacturing organizations (CDMOs) have resulted in production delays of 18-24 months, impairing timely clinical progress. Larger firms are investing in in-house production capacity, but smaller biotech companies remain dependent on external vendors, limiting their output control and increasing costs. Balancing therapeutic efficacy with immunogenicity in manufacturing processes adds further challenges, potentially decelerating market expansion.
The emerging economies of Asia Pacific and Latin America are investing heavily in genomics infrastructure, creating substantial growth opportunities. Asia Pacific, led by China and India, is projected to grow at a CAGR of 17.8% from 2025 to 2030, aided by government initiatives and the increasing prevalence of genetic disorders. The application of genome editing in crop genetics, improving yield, disease resistance, and reducing environmental impact, represents a lucrative market segment projected to augment overall market valuation substantially.
The development of base editing, prime editing, and microbiome editing platforms is providing new avenues for therapeutic innovation and commercial expansion. For example, startups focusing on phage-delivered CRISPR systems targeting antibiotic-resistant bacteria have attracted significant venture funding, highlighting an unmet need in infectious disease management. Incorporating these platforms into pharmaceutical pipelines promises to broaden the therapeutic scope beyond genetic diseases to complex disorders, thereby increasing market penetration.
Collaborative activities among academic institutions, biotech startups, and pharmaceutical companies are intensifying, accelerating technology transfer and commercialization. Licensing agreements, such as Precision BioSciences’ non-exclusive license to Caribou Biosciences for gene insertion technologies, are facilitating access to proprietary platforms and expanding product offerings. These partnerships not only foster innovation but also lower development costs and risks, thereby enhancing investor confidence and accelerating market growth trajectories.
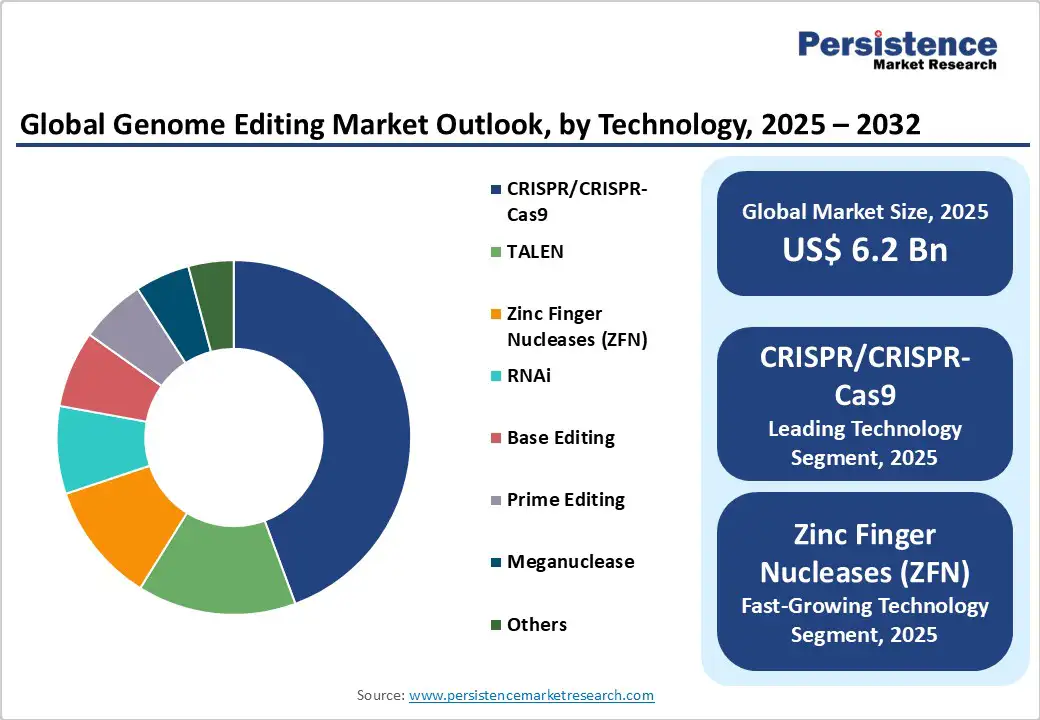
The CRISPR/Cas9 segment is slated to dominate with 44.7% market share in 2025 due to its high precision, adaptability, and cost-effectiveness, serving broad applications from gene knockouts to precise insertions. Continuous improvements in Cas9 variants and delivery methods enhance its clinical utility and adoption rates.
The fastest-growing technology segment is zinc finger nucleases (ZFN), projected to grow at the highest CAGR through 2032, driven by its lower off-target activity and increasing regulatory approvals for therapies targeting viral infections and cancers. Other emerging technologies, such as base and prime editing, are gaining traction due to enhanced accuracy and therapeutic potential.
Genetic engineering is set to lead among applications, encompassing cell line, animal, and plant genetic engineering, with an approximate 38.3% market revenue share in 2025, reflecting the extensive usage in drug discovery, agriculture, and biotechnology research. The dominance of the segment is attributable to the versatility and expanding use of genetic engineering in developing transgenic models and therapeutic molecules.
Clinical applications, including diagnostic and therapeutic genome editing, represent the fastest-growing segment with a high CAGR through 2032, fueled by the rise of personalized medicine and expanding clinical trial pipelines addressing genetic and chronic diseases.
In-vivo genome editing is likely to lead with around 60.5% genome editing market revenue share in 2025, particularly due to challenges in manipulating specialized cells ex-vivo and the rise of advanced delivery systems such as lipid nanoparticles and adeno-associated viruses (AAVs). It facilitates direct editing in the patient's body, expanding therapeutic possibilities for liver, neurological, and cardiovascular diseases.
The ex-vivo method is expected to grow at a high CAGR from 2025 to 2032, favored for its precise control over gene modification in isolated cells, notably in CAR-T cell therapies and hematopoietic stem cell editing, enabling safer and more efficient treatments.
Pharmaceutical and biotechnology companies are projected to constitute around 51.1% of the market in 2025, as these entities leverage genome editing extensively for novel therapeutic development, consolidated by collaborations with technology providers. This segment is the revenue leader due to investment in R&D and strategic partnerships.
Academic and research institutions represent the fastest-growing end-user group, forecasted to exhibit a high CAGR during 2025-2032, reflecting increasing incorporation of genome editing techniques in fundamental research, education, and early-stage therapeutic validation on multiple campuses and research labs globally.
North America is anticipated to dominate with approximately 44% of the market share in 2025, attributed to substantial R&D investments, strong biotech and pharma industries, and regulatory support fostering innovation. The U.S. particularly leads with major government funding and collaborative ecosystems accelerating clinical trial outputs.
Regulatory agencies are actively adapting policies to support genome editing therapeutics’ approval, while key players such as Thermo Fisher and Merck maintain manufacturing and distribution hubs here. Investment trends focus on clinical pipeline expansion, manufacturing capacity, and startup ecosystem support.
Europe is the second-largest regional market for genome editing, with approximately 24.4% of the market share, driven by countries such as Germany, the U.K., France, and Spain. The market in Germany benefits from the presence of key players such as Merck KGaA and active collaborations with global firms.
The U.K. fosters growth through supportive regulatory changes, such as the easing of field trial requirements for gene-edited plants. The regulatory environment established by the European Union (EU) is progressively harmonizing to facilitate genome editing development, while public and private funding initiatives boost R&D. Market competitiveness remains moderate, with notable mergers and technology partnerships shaping the landscape.
Asia Pacific is the fastest-growing regional market, expanding at the fastest CAGR through 2032, spearheaded by developments in China, Japan, India, and ASEAN countries. Regional growth is fueled by increasing healthcare infrastructure investments, government initiatives promoting precision medicine, and domestic companies such as GenScript leading adoption. Agricultural biotechnology advances and rising genomic research further stimulate demand. Manufacturing advantages and strategic investments by local firms are enhancing supply chain capabilities, while regulatory agencies progressively align with global standards to enable market penetration.

The global genome editing market landscape is moderately consolidated, with leading players holding approximately 45.0% combined market share. Key industry giants include Thermo Fisher Scientific, Danaher Corporation, Merck KGaA, and Takara Bio Inc., dominating through integrated product offerings and expansive distribution networks. The remaining market share is fragmented among numerous specialized startups focusing on niche gene editing platforms and applications. Competitive positioning revolves around technological innovation, strategic alliances, and vertical integration in manufacturing.
The genome editing market is projected to reach US$6.2 Billion in 2025.
The growing incidence of genetic disorders and cancer, coupled with advancements in genome editing technologies like CRISPR/Cas9, base editing, and prime editing, is propelling market growth.
The genome editing market is poised to witness a CAGR of 17.5% from 2025 to 2032.
The application of genome editing in crop genetics for improving yield, enhancing disease resistance, and reducing environmental impact, and the development of microbiome editing platforms for therapeutic innovation and commercial expansion are key market opportunities.
Thermo Fisher Scientific, Inc., Danaher Corporation, and Merck KGaA are some of the key players in the genome editing market.
| Report Attribute | Details |
|---|---|
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis |
Value: US$ Bn |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlight |
|
By Method
By Technology
By End-user
By Application
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author