ID: PMRREP35515| 198 Pages | 25 Jul 2025 | Format: PDF, Excel, PPT* | Healthcare

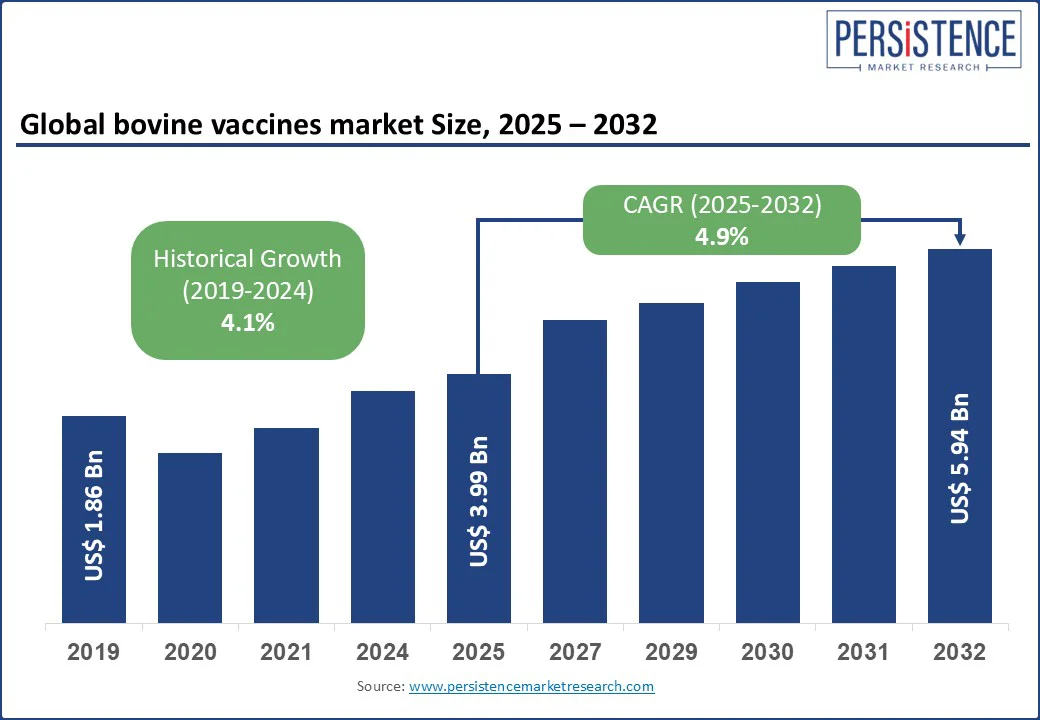
The global bovine vaccines market size is likely to be valued at US$ 3.99 Bn in 2025 and is expected to reach US$ 5.94 Bn by 2032, growing at a CAGR of 4.9% in the forecast period from 2025 to 2032. Rising demand for livestock health management and the increasing prevalence of infectious cattle diseases such as foot-and-mouth disease (FMD), bovine viral diarrhea (BVD), and brucellosis attract growth.
As the global meat and dairy industries expand, it drives the emphasis on preventive veterinary care to ensure animal productivity and food safety. Technological advancements in livestock vaccination solutions, including recombinant and DNA-based vaccines, are reshaping the market landscape, offering more effective and targeted immunization. The market is also benefiting from increasing investments by key players to develop cattle disease prevention products that address region-specific challenges and evolving pathogens.
Key Industry Highlights:
| Market Attribute | Key Insights |
| Bovine Vaccines Market Size (2025E) | US$ 3.99 Bn |
| Market Value Forecast (2032F) | US$ 5.94 Bn |
| Projected Growth (CAGR 2025 to 2032) | 4.9% |
| Historical Market Growth (CAGR 2019 to 2024) | 4.1% |
The rising regulatory mandates on curbing antimicrobial resistance are pushing farmers and regulators to shift from routine antibiotic use to precision cattle immunization strategies. This trend is most pronounced in North America, Europe, and Asia, where guidelines now favor vaccines over prophylactic antibiotics to address public health concerns. The clear return-on-investment from disease prevention vaccines is seen in reduced herd mortality, improved feed conversion, and lower cull rates. This drives both large-scale ranches and smallholders to adopt bovine vaccines proactively.
Innovations in thermostable vaccine formulations are alleviating cold-chain challenges in tropical and remote farming regions, improving access and reducing spoilage. Simultaneously, multivalent cattle vaccines that protect against multiple pathogens in a single dose are gaining traction by simplifying labor and lowering logistics costs. Moreover, DNA-based cattle vaccines are emerging as targeted, low-reactogenic solutions, strengthening herd immunity without compromising safety.
The steep investment in autogenous cattle vaccine development, including antigen customization and safety validation, escalates the cost per dose, far beyond standardized vaccines. Rigorous recombinant bovine vaccine manufacturing demands specialized facilities and highly skilled personnel, raising entry barriers and keeping prices high, particularly for emerging-market farmers. These production constraints hinder widespread adoption of advanced vaccines across price-sensitive rural regions, where cost remains a critical deterrent.
Maintaining thermostable vaccine integrity is challenging as cold-chain interruptions in remote regions often result in up to 30-40% dose spoilage, reducing farmer confidence and increasing per-dose cost. Meanwhile, heterogeneous regulatory approvals across countries inflate time-to-market for novel bovine vaccines, forcing manufacturers to navigate overlapping safety and licensing frameworks, delaying access, and increasing costs. These combined challenges slow the adoption of innovative formulations such as multivalent or DNA-based vaccines.
Manufacturers are capitalizing on the growing demand for autogenous cattle vaccines, which are custom-made using herd-specific strains to combat localized outbreaks such as lumpy skin disease. This tailor-made approach enhances efficacy in high-risk areas such as India and Southeast Asia, where farmers are increasingly seeking targeted biosecurity solutions. The evolution of recombinant and DNA-based bovine vaccines offers precise pathogen targeting with high safety profiles, accelerating uptake on commercial farms investing in cutting-edge immunization technologies.
Innovation in IoT-powered cold-chain modules such as ALIVE provides affordable, low-cost vaccine storage solutions, minimizing dose loss in remote farming regions and offering real-time temperature monitoring, opening expansion in underserved areas across Africa and Asia Pacific. Coupled with digital farm platforms, which integrate vaccine tracking, health monitoring, and scheduling, this technology empowers farmers with transparent livestock vaccination records, fueling adoption of advanced vaccines via digital trust-building.
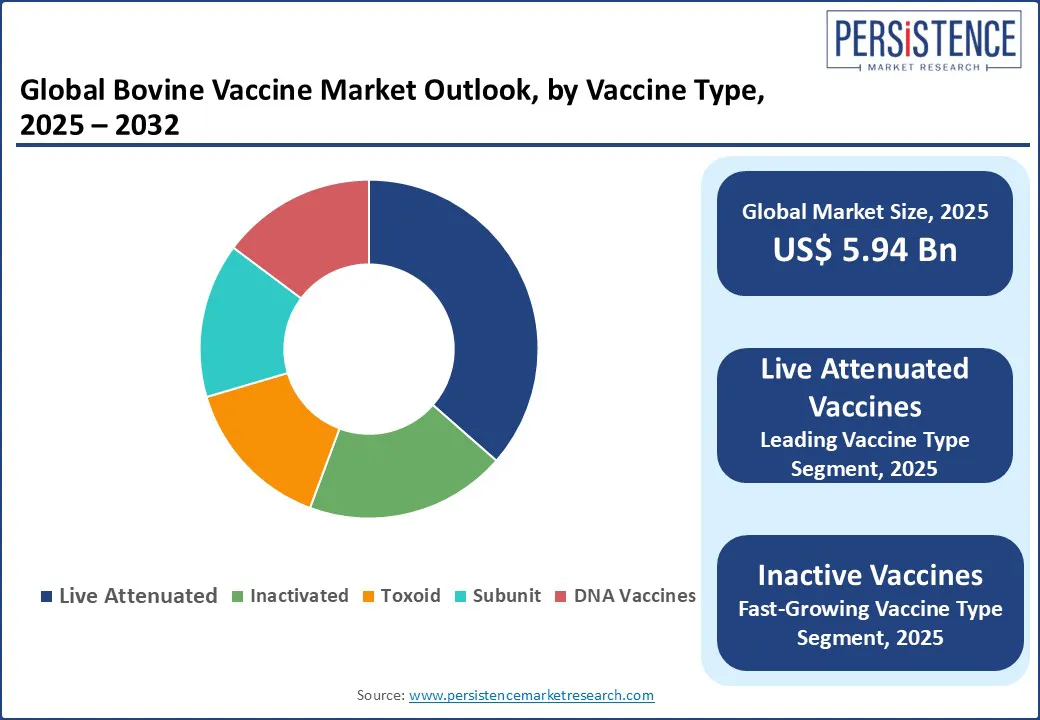
Live attenuated vaccines dominate with approximately 36.5% market share, driven by their ability to induce long-lasting immunity with just one or two doses and strong protection against diseases such as FMD, brucellosis, and BRD. Inactivated (Killed) Vaccines are favored for their safety profile and regulatory acceptance, and they are the fastest-growing segment due to improved efficacy and increased awareness. Subunit, Recombinant, and Toxoid Vaccines represent the evolving edge of bovine immunology. These vaccines utilize purified antigens or synthetic proteins to deliver targeted immune responses with minimal side effects.
BRD (Bovine Respiratory Disease Complex) leads disease-targeted vaccines with a 32% share, driven by high morbidity and the need for multivalent respiratory protection. The segment is also the fastest-growing, driven by the high economic burden per case and the expanding usage in feedlot operations globally. Mastitis, a leading cause of economic loss in the dairy sector, has become a central driver for vaccine investment, especially in regions where milk yield and quality are crucial market differentiators. Foot-and-Mouth Disease (FMD) remains a high-priority disease in many low- and middle-income countries, prompting aggressive national and regional vaccination campaigns.
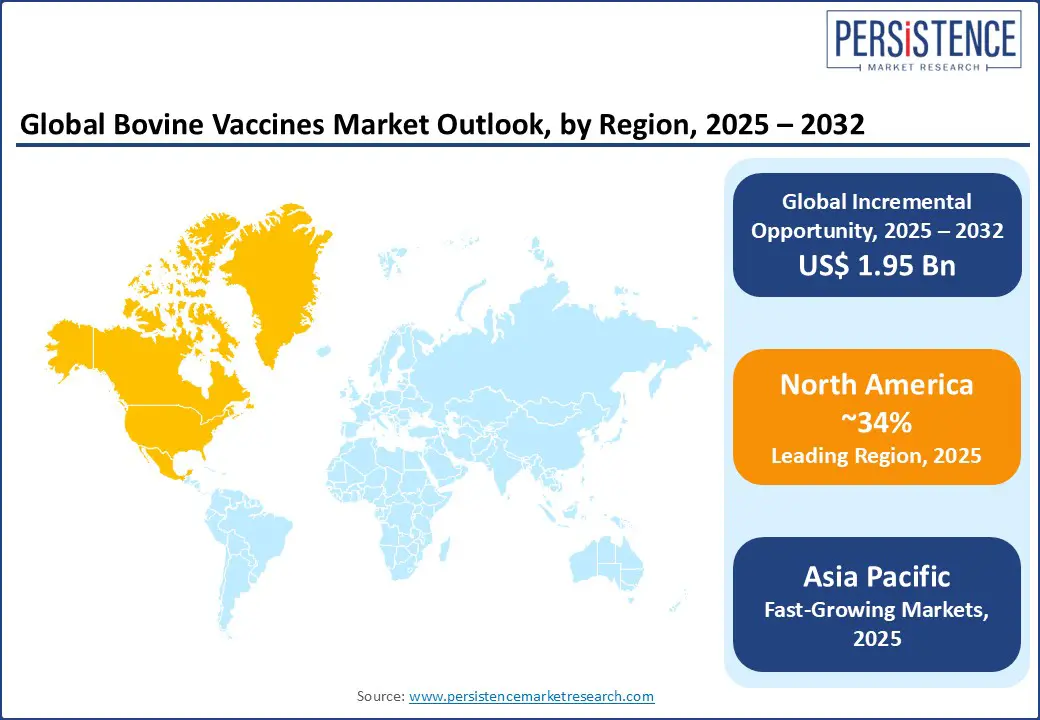
North America leads the global bovine vaccines market, capturing roughly 34% of the share thanks to its advanced veterinary healthcare system and strong government support. The region benefits from a highly structured veterinary healthcare system, strong regulatory oversight, and substantial government funding for livestock disease control.
In the U.S., the USDA and APHIS (Animal and Plant Health Inspection Service) have collaborated with private vaccine developers to ensure high standards for immunization programs. Bovine respiratory diseases and mastitis are the most targeted indications with widespread use of recombinant and DNA-based cattle vaccines among large-scale dairy and beef producers. Cold-chain integrity, traceability, and data-driven livestock health tracking are also well established in this region, enabling the effective delivery and monitoring of complex vaccination schedules. The rising emphasis on antibiotic-free animal husbandry further boosts demand for vaccines as a primary disease prevention tool.
Europe holds approximately 27% of the market. Its growth is driven by stringent animal welfare protocols and structured disease control programs. Countries such as Germany, France, the UK, and the Netherlands have long-standing vaccination mandates for diseases such as bovine viral diarrhea (BVD), bluetongue, and foot-and-mouth disease (FMD).
The EU's focus on food safety and animal traceability promotes the use of DIVA-compatible subunit and recombinant vaccines, especially in export-oriented cattle operations. Regional governments also invest in large-scale immunization campaigns in partnership with veterinary agencies. Europe is actively involved in the research and development of next-generation cattle vaccines, including thermostable and mRNA-based platforms, through collaborations between biotech firms and academic institutions. The continent also sees a growing trend toward needle-free cattle vaccine delivery systems and improved veterinary diagnostics to enhance disease surveillance.
Asia Pacific is experiencing the fastest growth, with a current market share of 22% and a projected CAGR of 7.8% during the forecast period. Rise in cattle population, growing awareness among livestock farmers, and proactive government-led immunization initiatives are some major growth factors. India and China lead the region, accounting for a significant portion of global bovine headcount.
In India, national programs such as the National Animal Disease Control Programme (NADCP) aim to eradicate FMD and brucellosis through sustained mass vaccination drives. Meanwhile, China continues to strengthen its animal health system to meet rising protein demands and biosecurity standards. Southeast Asian countries such as Indonesia, Vietnam, and Thailand are also expanding cattle vaccination coverage, particularly for zoonotic diseases. However, infrastructural gaps, cold-chain limitations, and lack of trained veterinary staff remain challenges. Despite this, the demand for thermostable, multivalent, and low-cost cattle vaccines is surging, opening opportunities for both local manufacturers and multinational players.
The global bovine vaccines market is highly competitive, led by established pharmaceutical giants and supported by emerging regional players. Companies such as Zoetis Inc., Merck Animal Health, and Boehringer Ingelheim Animal Health dominate due to their extensive product portfolios, global distribution networks, and investments in next-generation vaccine technologies. These companies also focus on value-added services such as vaccine scheduling, traceability, and diagnostics to maintain long-term relationships with large cattle farms and veterinary networks. Reflecting a widespread transformation, the market is moving toward thermostable formulations, autogenous vaccines, and digital integration, reflecting the industry’s response to region-specific challenges and demand for precise, scalable, and affordable cattle immunization solutions.
Key Industry Developments:
The global bovine vaccines market is expected to be valued at approximately US$ 3.99 Bn in 2025, driven by rising demand for preventive livestock care and large-scale immunization programs.
By 2032, the bovine vaccines market is projected to reach around US$ 5.94, reflecting growing adoption of advanced vaccines and increased government-led disease eradication efforts.
Key trends include the rise of thermostable and recombinant vaccines, mRNA and subunit-based innovations, increasing use of DIVA-compliant vaccines, and integration of digital herd health platforms to track vaccine efficacy and livestock immunity.
Among all segments, live attenuated vaccines dominate by vaccine type, while bovine respiratory disease (BRD) leads by disease type due to its high prevalence and economic impact on the global cattle industry.
The bovine vaccines market is expected to grow at a CAGR of 4.9% from 2025 to 2032, driven by increasing disease outbreaks, expanding commercial cattle farming, and enhanced veterinary infrastructure in emerging economies.
Major players with strong product portfolios and global reach include Zoetis Inc., Merck Animal Health, Boehringer Ingelheim Animal Health, Ceva Santé Animale, Indian Immunologicals Ltd.
| Report Attribute | Details |
| Historical Data/Actuals | 2019 - 2024 |
| Forecast Period | 2025 - 2032 |
| Market Analysis | Value: US$ Bn/Mn, Volume: As Applicable |
| Geographical Coverage |
|
| Segmental Coverage |
|
| Competitive Analysis |
|
| Report Highlights |
|
| Customization and Pricing | Available upon request |
By Vaccine Type
By Disease Type
By End-user
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author