ID: PMRREP33145| 552 Pages | 19 Jan 2026 | Format: PDF, Excel, PPT* | Healthcare

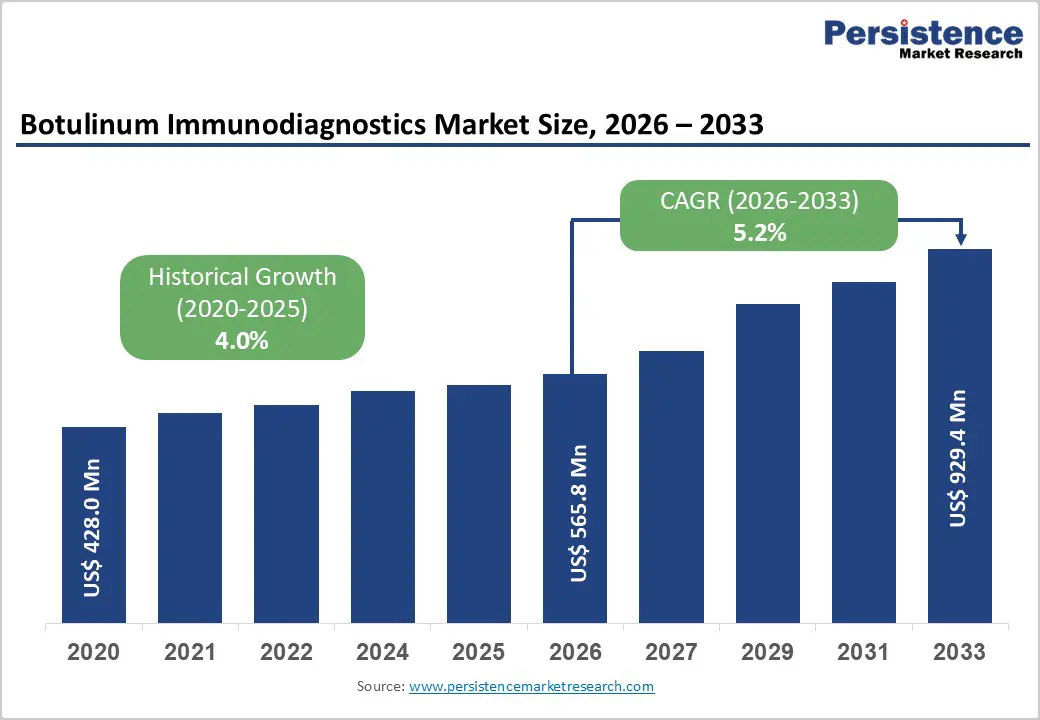
The global Botulinum Immunodiagnostics Market size is estimated to grow from US$ 565.8 Mn in 2026 to US$ 929.4 Mn by Mn 2033. The market is projected to record a CAGR of 5.2% during the forecast period from 2026 to 2033.
Global demand for botulinum immunodiagnostics is rising steadily, driven by increasing awareness of botulism as a critical neurological condition, heightened food safety surveillance, and growing public health preparedness for toxin-related outbreaks. Rising hospitalization rates linked to acute neuromuscular disorders, suspected foodborne illnesses, and infant botulism cases are increasing diagnostic testing volumes across hospitals and reference laboratories. Greater understanding of the extreme potency of botulinum toxin and its rapid clinical progression is reinforcing the need for early, accurate, and sensitive detection methods. Immunoassay-based diagnostics are gaining preference due to their reliability, scalability, and suitability for routine and emergency testing. Additionally, stringent regulatory oversight for toxin monitoring in food processing, pharmaceutical research, and biodefense programs is sustaining consistent demand for validated immunodiagnostic platforms. Expansion of laboratory automation, improvements in assay sensitivity, and integration with standardized workflows are enhancing testing efficiency. Growing investments in diagnostic infrastructure, rising healthcare expenditure, and increasing research activity in emerging economies are further supporting long-term global market growth.
| Global Market Attributes | Key Insights |
|---|---|
| Botulinum Immunodiagnostics Market Size (2026E) | US$ 565.8 Mn |
| Market Value Forecast (2033F) | US$ 929.4 Mn |
| Projected Growth (CAGR 2026 to 2033) | 5.2% |
| Historical Market Growth (CAGR 2020 to 2025) | 4.0% |
Driver – Increasing Botulism Surveillance, Food Safety Monitoring, and Biothreat Preparedness Supporting Diagnostic Demand
Rising global awareness of botulism as a rare but potentially fatal neuroparalytic condition is strengthening demand for reliable botulinum immunodiagnostics across clinical and public health systems. Botulinum toxin, produced by Clostridium botulinum, is among the most potent biological toxins known, requiring rapid and highly sensitive detection to support timely clinical intervention and outbreak control. Growing emphasis on early diagnosis in suspected foodborne, infant, and wound botulism cases is increasing routine utilization of immunoassay-based detection methods in hospitals and reference laboratories.
Additonally, food safety monitoring programs are expanding globally, particularly in processed food, dairy, and canned product supply chains, where botulinum contamination poses serious health risks. Regulatory authorities increasingly mandate preventive testing and surveillance, reinforcing the need for standardized diagnostic tools. In parallel, national biodefense and biopreparedness initiatives continue to classify botulinum toxin as a high-priority threat agent, driving sustained investment in detection capabilities. Technological improvements in immunoassay sensitivity, specificity, and automation compatibility are enhancing laboratory efficiency and accuracy. Collectively, heightened surveillance requirements, public health preparedness initiatives, and advances in assay performance are sustaining strong growth momentum for botulinum immunodiagnostics worldwide.
Restraints – Regulatory Complexity, Assay Sensitivity Challenges, and High Testing Costs Constraining Wider Adoption
The adoption of botulinum immunodiagnostics remains constrained by several technical and regulatory challenges. Diagnostic assays intended for botulinum toxin detection must meet exceptionally stringent regulatory standards due to the toxin’s extreme potency and public health implications. Validation requirements for sensitivity, specificity, and reproducibility often extend development timelines and increase compliance costs for manufacturers. Differences in toxin serotypes and complex sample matrices further complicate assay standardization, potentially affecting cross-reactivity and detection consistency.
Technical limitations also persist in distinguishing active toxin from inactive fragments, which can impact clinical interpretation and decision-making. While immunoassays offer significant advantages in speed and scalability, they continue to face competition from established reference methods that remain deeply embedded in regulatory and confirmatory testing frameworks. Cost-related factors add another layer of constraint, particularly for laboratories with limited budgets. Proprietary reagents, specialized consumables, and automation-compatible platforms can represent a significant financial burden for smaller hospitals, veterinary clinics, and laboratories in low-resource settings. Additionally, limited technical expertise and infrastructure gaps in developing regions restrict rapid adoption, moderating overall market penetration despite rising awareness of botulinum-related risks.
Opportunity – Growth in Rapid Diagnostics, Decentralized Testing, and Emerging Market Infrastructure Creating Expansion Potential
Significant growth opportunities are emerging as diagnostic technologies evolve toward faster, more accessible, and decentralized testing solutions. Development of rapid immunoassays, including lateral flow formats and compact analyzer-integrated platforms, is expanding the potential use of botulinum diagnostics beyond centralized reference laboratories. These technologies enable quicker turnaround times, supporting timely clinical decision-making during suspected botulism cases and outbreak investigations. Integration of digital readouts, automation, and data connectivity further enhances laboratory efficiency and surveillance capabilities.
Emerging economies across Asia Pacific, Latin America, and the Middle East present substantial untapped potential. Rising healthcare expenditure, expanding hospital networks, and increasing investment in food safety and public health infrastructure are improving access to advanced diagnostic tools. Governments in these regions are gradually strengthening regulatory frameworks and aligning testing standards with international guidelines, encouraging adoption of validated immunodiagnostic assays. Additionally, expanding academic research into neurotoxins, host–pathogen interactions, and microbial pathogenesis is sustaining research-based demand. Strategic collaborations between global diagnostic manufacturers, regional distributors, and public health agencies are expected to accelerate market entry and localization, positioning botulinum immunodiagnostics for sustained long-term growth.
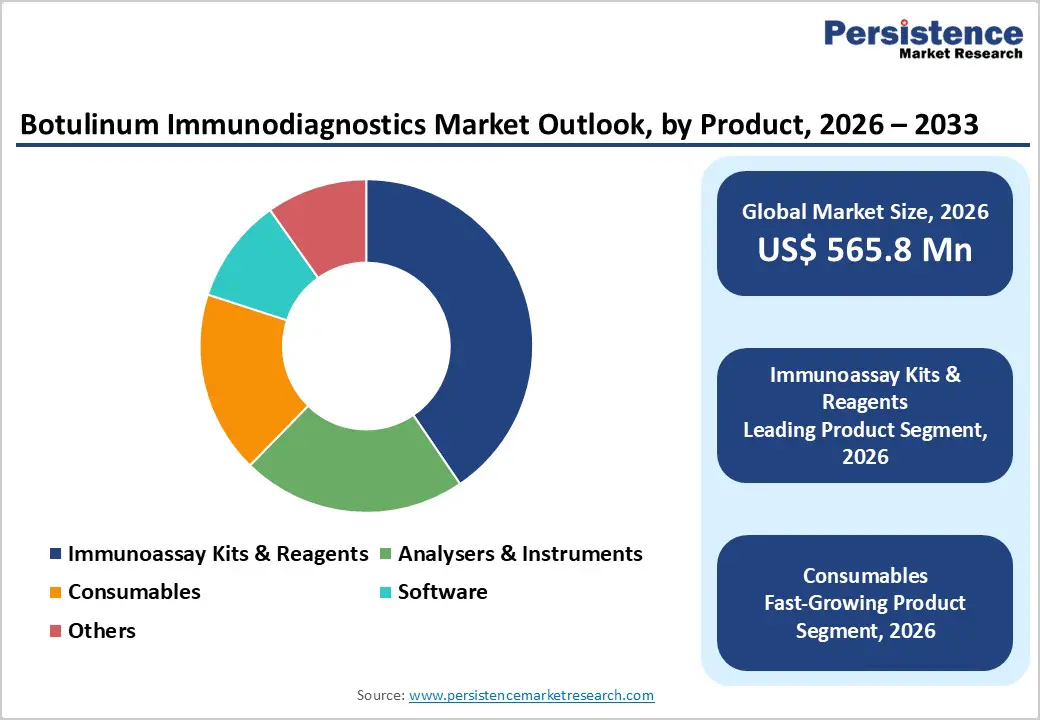
By Product, Immunoassay Kits & Reagents Lead Due to Recurring Demand and Broad Diagnostic Utility
The immunoassay kits & reagents segment is projected to dominate the global botulinum immunodiagnostics market in 2026, accounting for a revenue share of 40.5%. Segment leadership is primarily attributed to the recurring nature of reagent consumption in botulinum toxin detection across clinical diagnostics, food safety testing, biodefense surveillance, and research applications. Unlike analyzers and instruments, immunoassay kits are consumed with every testing cycle, ensuring consistent repeat purchasing and stable revenue streams. Their broad compatibility with commonly used platforms such as ELISA, fluorescence-based assays, and chemiluminescent immunoassays supports widespread adoption across hospital laboratories, public health agencies, and academic research centers. Continuous advancements in assay sensitivity, specificity, and lot-to-lot consistency are improving detection accuracy in complex biological and environmental samples. In parallel, growing preference for standardized, ready-to-use kits that minimize manual errors and reduce turnaround time is strengthening demand. Expansion of regulated testing requirements and increased preparedness for toxin-related outbreaks continue to reinforce the dominance of kits and reagents.
By Application, Clinical Diagnostics Dominates Due to Standardized Testing and Regulatory Acceptance
The clinical diagnostics segment is expected to lead the global botulinum immunodiagnostics market in 2026, capturing a revenue share of 30.3%. Leadership is supported by the critical need for rapid, accurate detection of botulinum toxin in suspected clinical cases, particularly in emergency and intensive care settings. Immunoassay-based diagnostic methods are widely accepted in clinical workflows due to their reliability, reproducibility, and compatibility with standardized laboratory protocols. Hospitals and reference laboratories rely on these assays for timely confirmation of botulism to support clinical decision-making and public health interventions. The availability of validated commercial immunodiagnostic kits, along with their compatibility with automated analyzers, enhances testing efficiency and scalability. Regulatory acceptance of immunoassay methodologies further supports adoption across healthcare systems. In addition, increasing awareness of foodborne and infant botulism, coupled with strengthened surveillance programs, is driving routine diagnostic testing. Strong familiarity among laboratory professionals and extensive historical performance data continue to position clinical diagnostics as the leading application segment.
By End User, Hospitals Lead Due to High Diagnostic Volumes and Critical Care Requirements
The hospitals segment is projected to dominate the botulinum immunodiagnostics market in 2026, accounting for a revenue share of 32.1%. Leadership is driven by the concentration of acute care services and the need for rapid diagnostic confirmation in suspected botulism cases. Hospitals serve as the primary point of care for severe neuromuscular and toxin-related conditions, requiring timely laboratory support to guide treatment decisions. High patient inflow, especially in emergency departments and intensive care units, sustains strong demand for immunodiagnostic testing. Hospitals are equipped with advanced laboratory infrastructure, trained personnel, and integrated diagnostic platforms that enable consistent utilization of immunoassay-based tests. In addition, hospital laboratories often function as regional referral centers, handling samples from surrounding clinics and public health authorities. Increasing emphasis on patient safety, early diagnosis, and outbreak preparedness further reinforces testing volumes. Regulatory compliance requirements and internal quality control protocols also support routine adoption of validated botulinum immunodiagnostic assays within hospital settings.
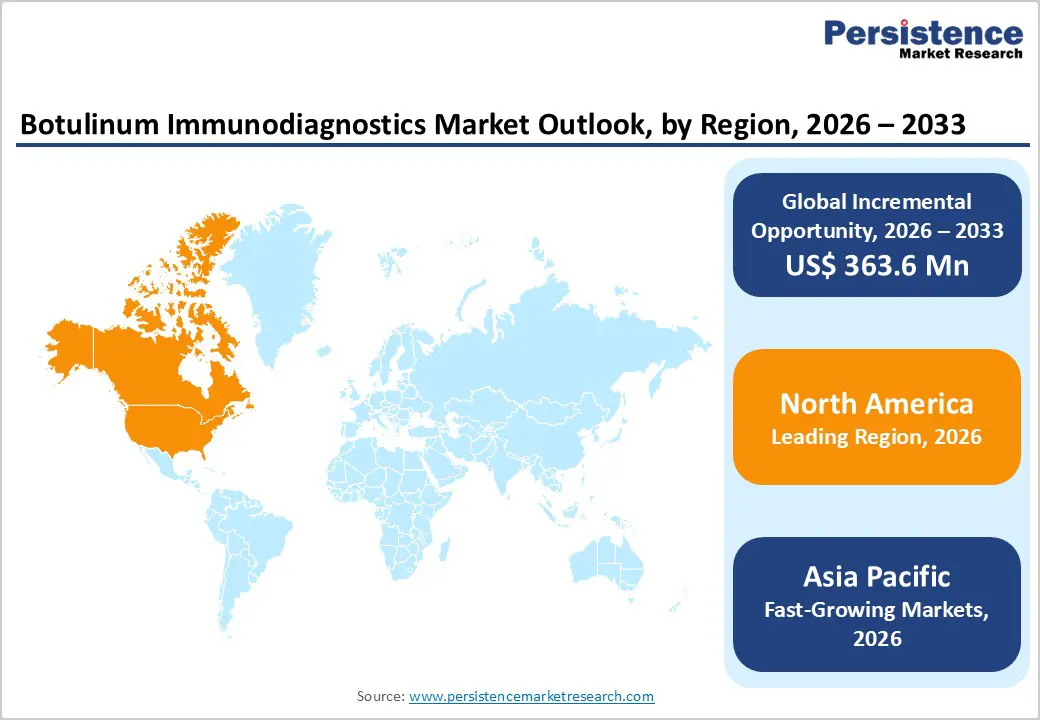
North America Botulinum Immunodiagnostics Market Trends
The North America botulinum immunodiagnostics market is expected to dominate globally with a value share of 48.5% in 2026, led primarily by the United States. Regional leadership is underpinned by advanced healthcare infrastructure, well-established laboratory networks, and high awareness of botulism as a public health concern. North America maintains robust surveillance systems for foodborne illnesses and biothreat agents, driving consistent demand for botulinum toxin detection assays across clinical and public health laboratories. Strong regulatory oversight by agencies such as the U.S. FDA and CDC promotes standardized diagnostic practices and validated immunoassay usage.
The region also benefits from significant biopharmaceutical and biotechnology activity, where botulinum toxin handling and safety monitoring require reliable diagnostic tools. High investments in research, biodefense preparedness programs, and academic–industry collaborations further support market growth. Early adoption of automated, high-sensitivity immunodiagnostic platforms enhances testing efficiency and accuracy. Presence of leading diagnostic manufacturers, favorable reimbursement structures, and continuous innovation collectively sustain North America’s dominant market position.
Europe Botulinum Immunodiagnostics Market Trends
The Europe botulinum immunodiagnostics market is expected to grow steadily, supported by strong regulatory frameworks, expanding diagnostic infrastructure, and heightened focus on infection control and food safety. Key countries including Germany, the U.K., France, Italy, and the Nordic nations contribute significantly due to their well-developed hospital systems and public health laboratories. European regulatory authorities enforce strict quality and safety standards for toxin detection, encouraging consistent adoption of validated immunoassay solutions. Increasing awareness of rare but severe neurological conditions such as botulism is driving diagnostic preparedness across healthcare facilities.
The region also benefits from strong academic research activity in microbiology and immunology, sustaining demand from research institutions. Growth in centralized laboratory services and gradual integration of automated immunodiagnostic platforms are improving testing throughput and reliability. Additionally, cross-border collaboration in disease surveillance and emergency response strengthens long-term demand. Expansion of pharmaceutical research and biologics development further reinforces the need for reliable botulinum toxin detection, supporting stable market growth across Europe.
Asia Pacific Botulinum Immunodiagnostics Market Trends
The Asia Pacific botulinum immunodiagnostics market is expected to register a relatively higher CAGR of around 7.1% between 2026 and 2033, driven by rapid healthcare expansion, increasing diagnostic capacity, and rising awareness of infectious and toxin-related diseases. Countries such as China, India, Japan, South Korea, and several Southeast Asian nations are investing heavily in hospital infrastructure and laboratory modernization. Improving access to diagnostic services is expanding the use of immunoassay-based toxin detection in both urban and semi-urban healthcare settings.
The region is also witnessing growth in pharmaceutical manufacturing, vaccine development, and food processing industries, all of which require robust safety and toxin monitoring systems. Gradual alignment of regional regulatory standards with international guidelines is supporting adoption of standardized immunodiagnostic assays. Increased government focus on disease surveillance, outbreak preparedness, and food safety is further strengthening demand. Growing collaborations with global diagnostic manufacturers and rising technical expertise are expected to sustain strong long-term market growth across Asia Pacific.
The global botulinum immunodiagnostics market is characterized by moderate to high competition, with established players such as Thermo Fisher Scientific Inc., Bio-Techne, BioChek, AdVnt Biotechnologies, and Response Biomedical holding strong positions. These companies benefit from robust immunodiagnostic portfolios, specialized expertise in toxin detection, and well-established distribution networks serving clinical laboratories, research institutions, food safety agencies, and public health organizations.
Competitive strategies emphasize enhancements in assay sensitivity, specificity, and rapid-testing capabilities, alongside improved compatibility with automated laboratory platforms. Market participants are also focused on geographic expansion, particularly in emerging economies, while prioritizing regulatory approvals, sustained R&D investment, and strategic partnerships to reinforce market presence and support long-term growth.
The global botulinum immunodiagnostics market is projected to be valued at US$ 565.8 Mn in 2026.
The global botulinum immunodiagnostics market is driven by technological advancements in high-sensitivity immunoassays, rising awareness of botulism risks including food safety and biothreat preparedness, and increasing demand for rapid and accurate diagnostic tools in clinical, environmental, and food testing settings.
The global botulinum immunodiagnostics market is poised to witness a CAGR of 5.2% between 2026 and 2033.
Significant opportunities exist in developing rapid, low-cost diagnostic kits and expanding into emerging regions with growing healthcare infrastructure and food safety demand.
Thermo Fisher Scientific Inc., Bio-Techne, BioChek, AdVnt Biotechnologies, and Response Biomedical are some of the key players in the botulinum immunodiagnostics market.
| Report Attributes | Details |
|---|---|
| Historical Data/Actuals | 2020 – 2025 |
| Forecast Period | 2026 – 2033 |
| Market Analysis | Value: US$ Mn Volume (Units) If Applicable |
| Geographical Coverage |
|
| Segmental Coverage |
|
| Competitive Analysis |
|
| Report Highlights |
|
By Product
By Application
By End User
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author