ID: PMRREP34958| 174 Pages | 26 Aug 2025 | Format: PDF, Excel, PPT* | Healthcare

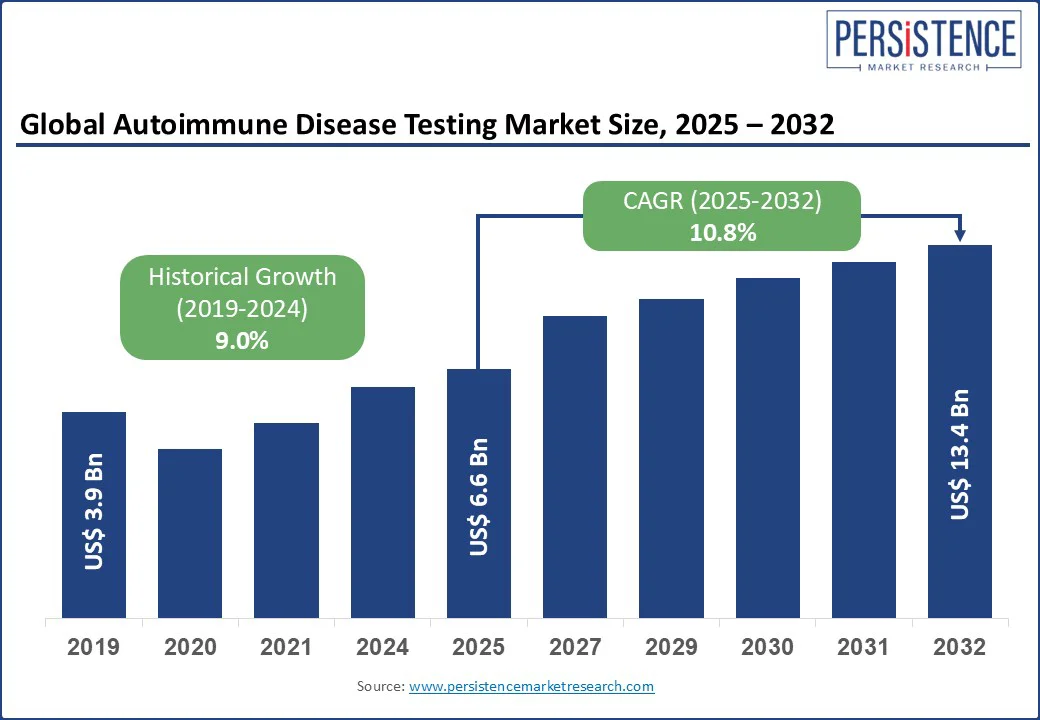
The global autoimmune disease testing market size is likely to be valued at US$6.6 Bn in 2025 and reach US$13.4 Bn by 2032, growing at a CAGR of 10.8% by 2032, during the forecast period from 2025 to 2032.
The increasing prevalence of chronic autoimmune disorders such as systemic lupus erythematosus, Hashimoto’s thyroiditis, and celiac disease fuels the need for autoimmune disease testing. Technological advancements in ELISA, CLIA, IFA, PCR, and multiplex immunoassays follow demand for early diagnosis, personalized medicine, and point-of-care testing.
Key Industry Highlights:
|
Global Market Attribute |
Key Insights |
|
Autoimmune Disease Testing Market Size (2025E) |
US$6.6 Bn |
|
Market Value Forecast (2032F) |
US$13.4 Bn |
|
Projected Growth (CAGR 2025 to 2032) |
10.8% |
|
Historical Market Growth (CAGR 2019 to 2024) |
9.0% |
The global surge in autoimmune diseases, such as rheumatoid arthritis, Systemic lupus erythematosus (SLE), Type 1 diabetes, and inflammatory bowel disease, is significantly driving demand for accurate and early diagnostic testing. Increasing awareness and adoption of advanced ELISA, chemiluminescence immunoassay (CLIA), and multiplex immunoassay platforms are enabling faster diagnosis and effective disease monitoring.
Hospitals and clinical laboratories are increasingly deploying autoantibody panels, ANA, anti-CCP, and HLA typing kits, improving diagnostic throughput and accuracy. Government-led screening programs and favourable reimbursement policies in regions such as North America and Europe further reinforce market growth.
Despite technological advancements, the market faces challenges due to the lack of standardized diagnostic protocols and limited access to specialized testing infrastructure in many developing regions. Variability in test results, especially in IFA (Indirect Fluorescent Antibody) tests and immunoblotting, leads to diagnostic ambiguity.
In low-resource settings, limited awareness among primary care physicians, high costs of multiplex tests, and poor reimbursement coverage restrict market penetration. Additionally, the asymptomatic nature of early autoimmune diseases makes timely detection difficult without routine screening.
The rising demand for personalized medicine and early disease screening presents a major opportunity for market players to develop rapid, affordable, and portable diagnostic kits. Advancements in biosensors, flow cytometry, PCR-based panels, and AI-assisted interpretation tools are creating pathways for point-of-care autoimmune testing in both urban and rural healthcare settings.
Companies can also tap into the unmet needs in Asia Pacific and Middle Eastern countries, where the incidence of autoimmune diseases is growing, but testing infrastructure remains underdeveloped. Partnerships with government agencies, mobile diagnostic networks, and telehealth platforms can further boost accessibility and market reach.
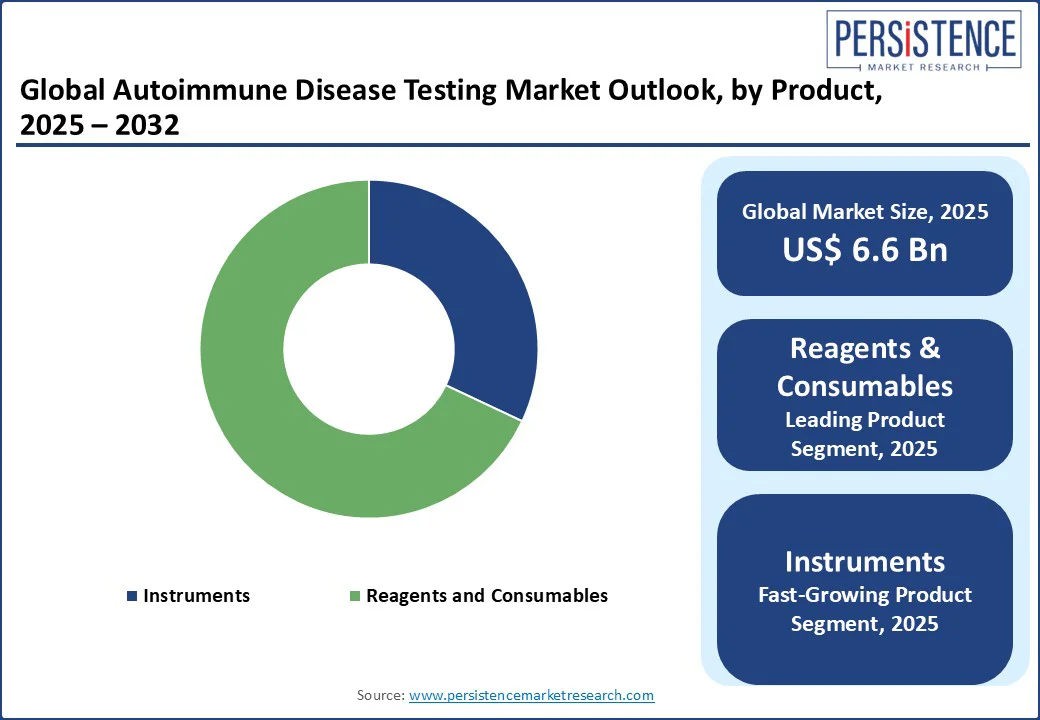
The reagents and consumables segment leads the autoimmune disease diagnostics market, holding a 68% revenue share in 2025. This dominance stems from their essential role in laboratory tests, including test kits and antibodies. The increasing prevalence of autoimmune disorders and advancements in biomarkers have boosted demand for specialized consumables.
Technological innovations have also made these products more accessible in resource-limited settings. Given the diverse nature of autoimmune diseases requiring various tests, the need for consumables continues to grow. Unlike longer-lasting instruments, consumables require regular replenishment, ensuring steady demand and reinforcing their position as the dominant product category in this market.
The localized autoimmune disease segment held the largest share of the autoimmune disease diagnostics market, accounting for 67% share in 2025. This dominance is driven by the high prevalence of localized autoimmune conditions, advancements in diagnostic technologies, growing awareness, and ongoing research initiatives.
Innovations such as antigen microarray and mass spectrometry have greatly enhanced antibody profiling, delivering improved analytical sensitivity and reproducibility. These cutting-edge techniques, with further refinement and validation, are expected to become part of routine clinical practice, further boosting diagnostic accuracy.
The rising adoption of advanced testing methods, along with increased screening rates for conditions like inflammatory bowel disease, type 1 diabetes, and thyroid disorders, has significantly contributed to market growth. Moreover, collaborative efforts between healthcare providers and researchers are enhancing early detection and patient management, solidifying the localized segment’s position as the leading contributor in the autoimmune disease diagnostics industry.
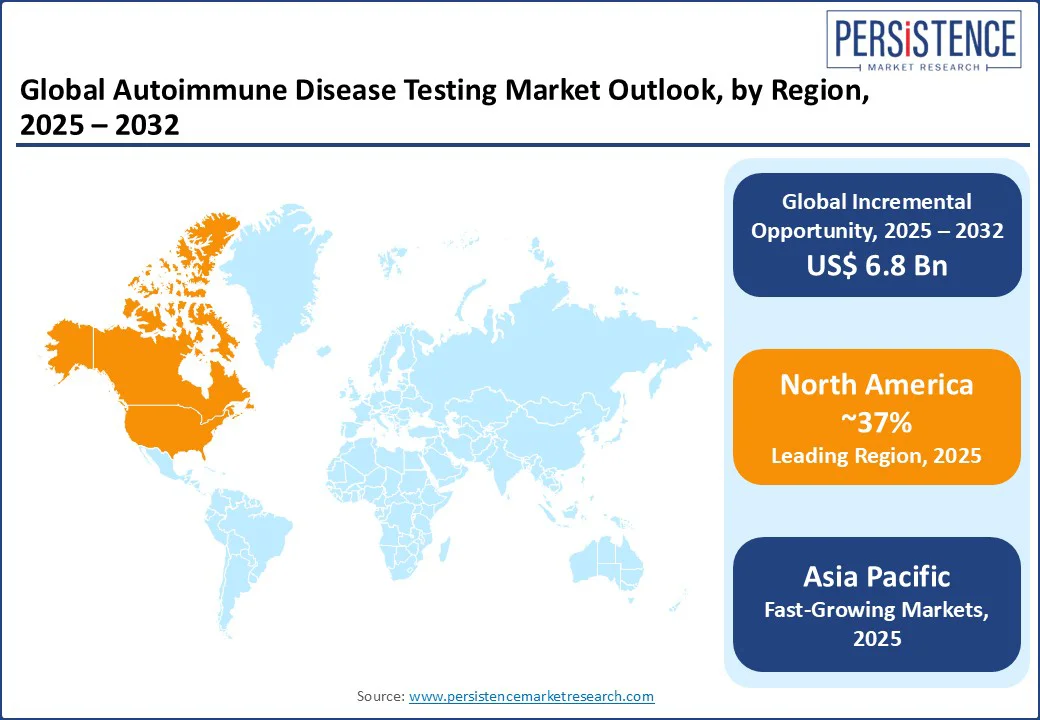
The autoimmune disease testing market in North America is expanding due to increasing prevalence of chronic autoimmune disorders, improved healthcare infrastructure, and a shift toward advanced technologies such as ELISA and multiplex immunoassays. Diagnostic providers are expanding their test menus to meet rising clinical demand and improve early detection and disease monitoring.
The rise of ELISA and chemiluminescence immunoassays in the U.S. is revolutionizing autoimmune disease testing with increased accuracy and volume. The CDC reveals that nearly 23.5 million Americans are affected by these conditions, emphasizing the need for effective diagnostics.
With ongoing research, supportive reimbursement, and heightened awareness, the future of autoimmune diagnostics in North American hospitals and labs looks promising. Exciting advancements are on the horizon.
Europe's autoimmune disease testing market is fueled by a growing elderly population, rising autoimmune disease burden, and increasing adoption of advanced diagnostic technologies like immunofluorescence and immunoblotting. Public healthcare systems across the region ensure wider access to diagnostic testing and promote earlier diagnosis through national screening and lab integration efforts.
Germany’s hospital laboratories, supported by companies such as Siemens Healthineers, have incorporated fully automated immunoassay platforms for testing. The UK has also expanded testing programs for celiac disease and lupus through the NHS and diagnostic partnerships with Euroimmun and Thermo Fisher Scientific.
Europe’s centralized healthcare model and investments in laboratory automation position the region as a strong growth hub for autoimmune diagnostics.
The autoimmune testing market in the Asia Pacific is accelerating due to the rising incidence of diseases like Type 1 diabetes, thyroiditis, and lupus, along with improved diagnostic infrastructure and awareness campaigns. Growth is further supported by technological integration, such as PCR, ELISA, and portable immunoassay kits for point-of-care testing in remote areas.
In India and China, companies like Mindray are launching rapid test kits and semi-automated analyzers targeting primary care centres. Japan’s national health programs are promoting routine autoimmune screenings, especially for thyroid and celiac disorders, using multiplex panels in hospitals and clinics.
Increasing public-private collaboration, affordability of test kits, and growing healthcare investment will continue to accelerate autoimmune testing adoption across the Asia Pacific.
The autoimmune disease testing market has a consolidated structure with a competitive environment. Market leaders maintain a commanding position with an array of autoimmune disease diagnostic equipment and supplies. They have a robust presence in the industry along with an extensive global distribution network and considerable knowledge in the healthcare and diagnostics sectors.
Other manufacturers in the industry actively engage in developing innovative products to expand their product portfolio. They are also exploring geographical expansion through mergers and acquisitions to gain market share.
The global market is set to reach US$ 6.6 Bn in 2025.
Autoimmune disease testing market is projected to reach CAGR of 10.8% during the forecast period from 2025 to 2032.
Demand for autoimmune disease testing services is growing globally due to the rising prevalence of autoimmune diseases and the adoption of advanced diagnostic technologies.
Abbott Laboratories, Thermo Fisher Scientific, F. Hoffmann-La Roche Ltd, Siemens Healthineers, Bio-Rad Laboratories, Beckman Coulter (Danaher Corporation), Quest Diagnostics, PerkinElmer, Inc., are a few leading players.
North America is expected to lead the global market in 2025, capturing a market share of 37%.
|
Report Attribute |
Details |
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis Units |
Value: US$ Bn Volume: Units |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
|
Customization and Pricing |
Available upon request |
By Product
By Disease Type
By Test Type
By End-user
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author