ID: PMRREP27115| 200 Pages | 6 Jan 2026 | Format: PDF, Excel, PPT* | Healthcare

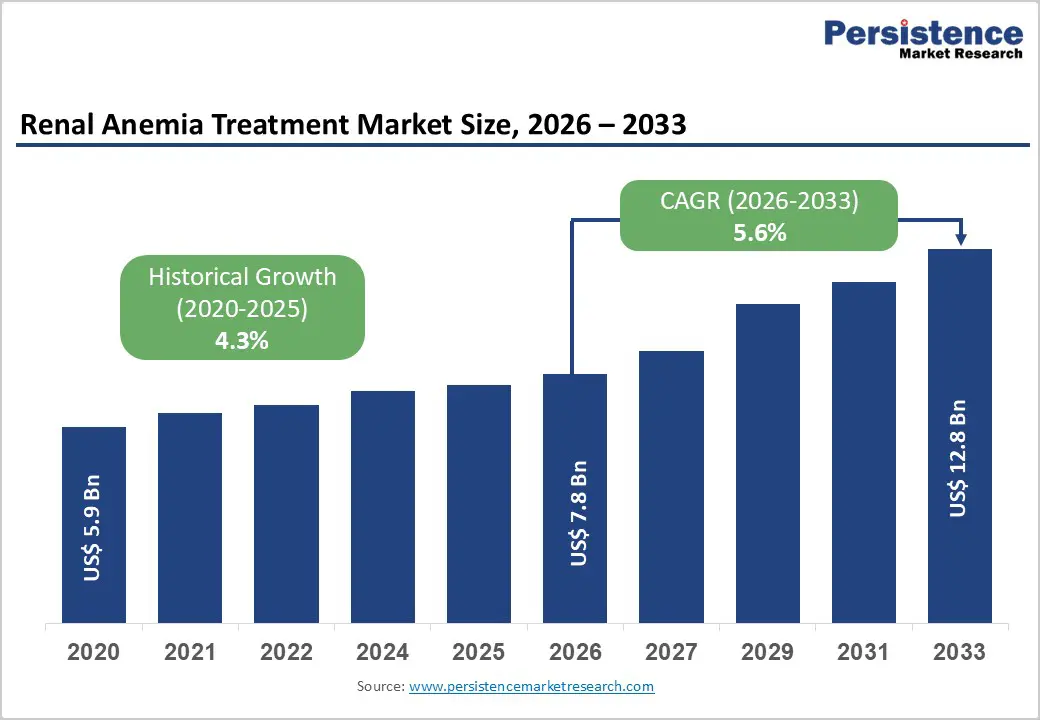
The global renal anemia treatment market size is estimated to grow from US$ 7.8 Bn in 2026 to US$ 12.8 Bn by 2033. The market is projected to record a CAGR of 5.6% during the forecast period from 2026 to 2033.
Global demand for renal anemia treatment is rising rapidly, driven by the growing global chronic kidney disease (CKD) burden, expanding dialysis population, and increasing clinical emphasis on early anemia correction to prevent CKD progression. Hospitals and specialty nephrology centers are increasingly utilizing ESAs, IV iron therapies, and emerging HIF-PH inhibitors to support routine CKD management, stabilize hemoglobin levels, and reduce transfusion dependence. Rising investments in renal-care infrastructure, expansion of dialysis networks, and the growth of integrated CKD management programs are accelerating global adoption. Continuous advancements in long-acting ESA formulations, oral HIF-PH inhibitors, improved iron therapeutics, and AI-enabled renal monitoring technologies are significantly improving treatment precision, patient convenience, and long-term outcomes. Additionally, growing adoption of guideline-based anemia protocols, increasing CKD prevalence linked to diabetes and hypertension, and expanding clinical evidence supporting novel therapeutics are further propelling global market growth.
| Global Market Attributes | Key Insights |
|---|---|
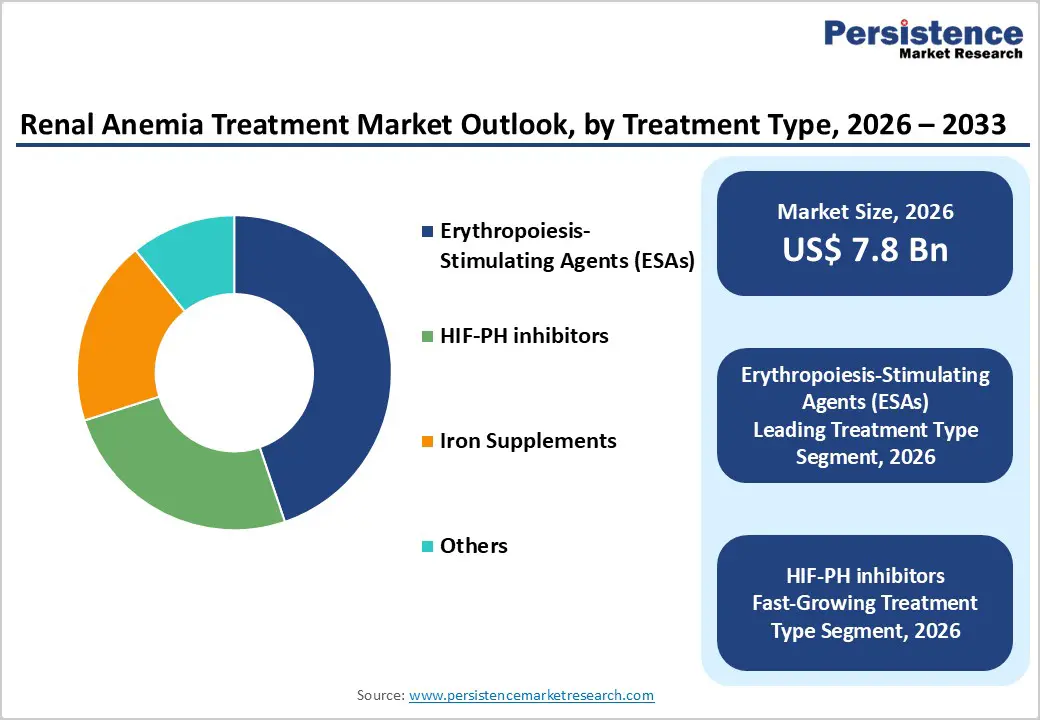
| Renal Anemia Treatment Market Size (2026E) | US$ 7.8 Bn |
| Market Value Forecast (2033F) | US$ 12.8 Bn |
| Projected Growth (CAGR 2026 to 2033) | 5.6% |
| Historical Market Growth (CAGR 2020 to 2025) | 4.3% |
The renal anemia treatment market is strongly propelled by the dual impact of the rising global chronic kidney disease (CKD) burden and rapid advancements in anemia therapeutics, particularly ESAs and HIF-PH inhibitors. CKD prevalence continues to climb due to escalating rates of diabetes, hypertension, obesity, and aging populations, significantly expanding the pool of patients requiring anemia management across stages 3-5 and dialysis settings. For instance, in 2023, the Institute for Health Metrics and Evaluation (IHME) estimated that global cases of kidney failure requiring treatment (KFRT) reached 4.59 million across all ages and both sexes, corresponding to an age-standardized prevalence of 50.7 cases per 100,000 population. As impaired erythropoietin production becomes more pronounced with CKD progression, demand for clinically effective correction therapies has intensified. In parallel, therapeutic innovation is reshaping treatment paradigms. Long-acting erythropoiesis-stimulating agents (ESAs) are improving dosing convenience, enhancing hemoglobin stability, and reducing the need for frequent injections in dialysis patients.
Additionally, orally administered hypoxia-inducible factor prolyl hydroxylase (HIF-PH) inhibitors are transforming treatment accessibility for non-dialysis CKD populations by offering an alternative to injectable ESAs, supporting better adherence and reduced transfusion dependency. Their mechanisms promote endogenous erythropoietin production, improve iron utilization, and address inflammation-associated anemia. Together, the accelerating CKD epidemic and expanding therapeutic landscape are driving sustained demand for advanced renal anemia interventions across global healthcare systems.
The renal anemia treatment market faces significant restraints due to the combined impact of high therapy costs and safety-related limitations associated with erythropoiesis-stimulating agents (ESAs). The premium pricing of biologic ESAs and newer oral HIF-PH inhibitors places a substantial financial burden on healthcare systems, particularly in low- and middle-income countries where reimbursement mechanisms are limited. As CKD prevalence rises, the cumulative cost of long-term anemia management often requiring lifelong therapy further strains budgets, reducing accessibility among underserved populations.
Moreover, concerns over cardiovascular risks linked to aggressive ESA dosing constrain clinical decision-making. Evidence suggesting heightened risks of stroke, thromboembolic events, and hypertension at higher hemoglobin targets has led to more conservative dosing protocols. These safety considerations limit physicians’ ability to escalate therapy, particularly in elderly patients and those with multiple comorbidities. As a result, many CKD patients remain undertreated or require therapy modifications that slow hemoglobin correction. Together, the dual challenge of high treatment costs and stringent safety constraints creates barriers to widespread adoption, particularly in resource-limited settings, and underscores the need for cost-effective alternatives and improved risk-stratified treatment algorithms.
The renal anemia treatment market is poised for strong growth as multiple high-value opportunities converge across therapeutic innovation, healthcare infrastructure development, and digital transformation. The growing adoption of oral HIF-PH inhibitors represents one of the most significant opportunities, particularly as these therapies increasingly penetrate the large non-dialysis CKD population that historically faced limited treatment options. Their oral administration, improved tolerability, and ability to stimulate endogenous erythropoietin production position them as a major driver of future market expansion.
Furthermore, emerging economies are witnessing rapid investment in dialysis infrastructure, driven by rising CKD prevalence, public-private partnerships, and government-supported renal-care programs. This expansion is increasingly translating into strong demand for ESAs, IV iron formulations, and supportive anemia-management protocols across new dialysis facilities. Complementing these advancements, the development of personalized and AI-driven anemia management platforms is unlocking a new frontier in precision nephrology. AI-enabled hemoglobin monitoring, predictive modeling, and adaptive dosing algorithms enhance treatment accuracy, reduce variability in patient response, and optimize resource utilization. Together, these therapeutic, infrastructural, and digital opportunities create a multi-dimensional growth landscape that will significantly boost the global renal anemia treatment market.
The erythropoiesis-stimulating agents (ESAs) segment is projected to dominate the global renal anemia treatment market in 2026, accounting for a significant revenue share of 44.80%. This leadership is driven by the widespread clinical adoption of ESAs as the standard of care for anemia associated with chronic kidney disease (CKD), particularly among dialysis and late-stage CKD patients. Their ability to effectively elevate hemoglobin levels, reduce transfusion requirements, and provide consistent dosing options supports broad utilization across hospital and outpatient nephrology settings. Continued innovation in long-acting ESA formulations, integration with dialysis workflows, and strong clinical guideline recommendations further accelerate adoption. Increasing CKD prevalence, expanding dialysis capacity, and long-term safety data continue to reinforce ESAs’ dominant position in global renal anemia management.
The normocytic anemia segment is projected to dominate the global renal anemia treatment market in 2026, accounting for a significant revenue share of 42.7%. This dominance stems from the fact that normocytic anemia is the most common anemia type associated with CKD, resulting from impaired erythropoietin production and chronic inflammation. Its widespread incidence across stages 3-5 CKD drives substantial clinical demand for ESAs, IV iron therapies, and emerging HIF-PH inhibitors. Consistent diagnosis through routine renal function testing, strong guideline-supported intervention strategies, and well-established treatment pathways further support higher adoption rates. Growing CKD patient populations, rising diabetes and hypertension prevalence, and early intervention emphasis in nephrology programs reinforce the strong market positioning of the normocytic anemia segment.
The hospitals segment is projected to dominate the global renal anemia treatment market in 2026, capturing a revenue share of 56.7%. Hospitals particularly tertiary-care centers, nephrology units, and academic medical institutions serve as primary hubs for renal anemia diagnosis and treatment due to their ability to manage complex CKD cases, administer ESAs and IV iron therapies, and support comprehensive dialysis services. High patient inflow, availability of specialized nephrologists, and integration of advanced renal-care technologies contribute to strong hospital-based demand. Additionally, hospitals play a central role in CKD screening, guideline-based anemia management, and clinical research evaluating emerging HIF-PH inhibitors and iron formulations. Their extensive capacity, multidisciplinary care environment, and critical role in managing end-stage renal disease solidify hospitals as the leading end-user segment globally.
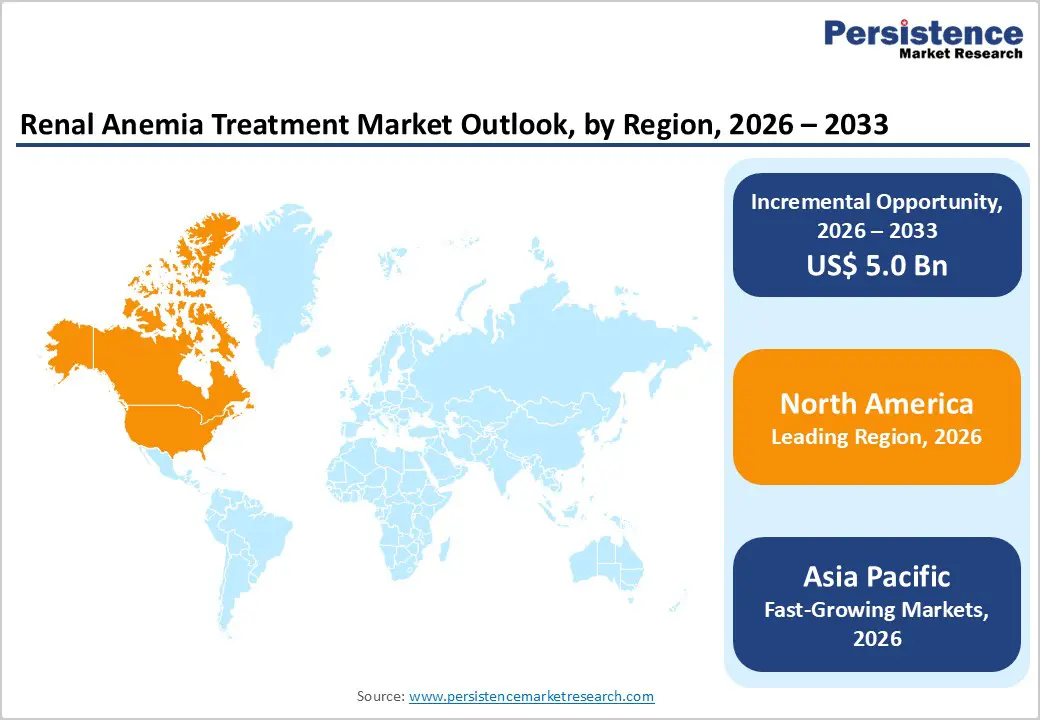
North America is expected to maintain global dominance in the renal anemia treatment market with a market share value of 46.3%, supported by its advanced healthcare infrastructure, strong CKD management programs, and widespread adoption of ESAs, IV iron therapies, and emerging HIF-PH inhibitors. The U.S. leads the region due to frequent FDA approvals of new renal anemia therapeutics, strong presence of leading biopharmaceutical manufacturers, and extensive collaborations between dialysis centers, hospitals, and academic research institutions. Major nephrology networks continuously evaluate new ESA formulations, IV iron products, and oral HIF-PH inhibitors, accelerating clinical integration. Rising demand for early CKD-stage anemia management and decentralized care delivery continues to drive investments from large healthcare systems.
The region also benefits from strong reimbursement frameworks for CKD and dialysis services, high clinician preference for long-acting ESAs and advanced iron therapies, and expanding adoption of oral HIF-PH inhibitors. Increasing investment in dialysis infrastructure, digital care coordination platforms, and AI-supported renal disease management tools is improving treatment efficiency. Additionally, rising public awareness of CKD progression and expanding nephrology research hubs continue to strengthen North America’s long-term leadership in the global renal anemia treatment market.
Europe shows steady and mature adoption of renal anemia treatment solutions, supported by strong public-health policies, well-established dialysis networks, and stringent clinical management guidelines across major markets such as Germany, the U.K., France, Italy, Switzerland, and the Nordic region. Robust epidemiological surveillance systems and consistent clinical validation of new therapies support the deployment of ESAs, IV iron formulations, and HIF-PH inhibitors across primary and specialty care. The region demonstrates high integration of automated drug delivery systems, standardized anemia protocols, and evidence-based CKD management pathways, driven by the need to improve hemoglobin stability and reduce transfusion dependency.
Europe’s favorable regulatory framework, emphasis on safety and efficacy, and strong contributions from clinical research centers support the evaluation of next-generation renal anemia therapeutics. Growing demand for cost-effective long-acting ESAs, improved iron therapies, and orally administered HIF-PH inhibitors continues to strengthen uptake. Regional pharmaceutical manufacturers are investing in formulation innovation, GMP-compliant production, and sustainable supply chains. Government initiatives supporting CKD screening, early anemia intervention, and digital treatment monitoring further drive Europe’s overall market growth.
Asia Pacific is projected to be the fastest-growing region for renal anemia treatment with a CAGR of 7.6%, driven by rising healthcare expenditure, increasing CKD burden, and rapid expansion of dialysis centers. Countries such as China, Japan, South Korea, Singapore, and India are increasing adoption of ESAs, IV iron therapies, and new HIF-PH inhibitors across hospitals, nephrology clinics, and government healthcare programs. Growing availability of cost-effective biologics and generic formulations, combined with strong participation from regional pharmaceutical manufacturers, is improving affordability and expanding access across mid-sized hospitals and community settings.
Government-supported CKD screening programs, investments in dialysis infrastructure, and partnerships with global biopharma firms for technology transfer are accelerating adoption. Increasing need for early anemia detection, streamlined CKD management, and oral therapy options is driving strong clinical uptake. Nephrologists across Asia Pacific are increasingly participating in global kidney disease research networks, adopting advanced treatment protocols to reduce disease progression. Expanding private healthcare networks, rising medical tourism, and the growth of specialized renal-care institutes continue to support robust market growth across the region.
The global renal anemia treatment market is highly competitive, with active participation from Johnson & Johnson, AstraZeneca, Amgen Inc., GSK plc., and Pfizer Inc. These companies leverage strong nephrology portfolios and extensive biologics capabilities to expand their presence across dialysis centers and hospital networks. Growing CKD prevalence continues to drive the adoption of ESAs, HIF-PH inhibitors, and advanced iron therapies.
Manufacturers are prioritizing long-acting ESAs, oral HIF-PH inhibitors, and enhanced iron formulations, while focusing on regulatory approvals, scaled production, and partnerships with healthcare systems to improve treatment access and support market expansion.
The global renal anemia treatment market is projected to be valued at US$ 7.8 Bn in 2026.
Rising chronic kidney disease (CKD) prevalence, expanding dialysis population, and increasing adoption of ESAs and novel HIF-PH inhibitors. drive the global renal anemia treatment market.
The global renal anemia treatment market is poised to witness a CAGR of 5.6% between 2026 and 2033.
Growing uptake of oral HIF-PH inhibitors, emerging CKD patient pools in developing regions, and innovation in long-acting anemia therapeutics are creating opportunities in the market.
Johnson & Johnson, AstraZeneca, Amgen Inc., GSK plc. and Pfizer Inc. are some of the key players in the renal anemia treatment market.
| Report Attributes | Details |
|---|---|
| Historical Data/Actuals | 2020 - 2025 |
| Forecast Period | 2026 - 2033 |
| Market Analysis | Value: US$ Bn; Volume (In Units) If applicable |
| Geographical Coverage |
|
| Segmental Coverage |
|
| Competitive Analysis |
|
| Report Highlights |
|
By Treatment Type
By Disease Type
By Route of Administration
By End User
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author