ID: PMRREP29971| 185 Pages | 8 Oct 2025 | Format: PDF, Excel, PPT* | Healthcare

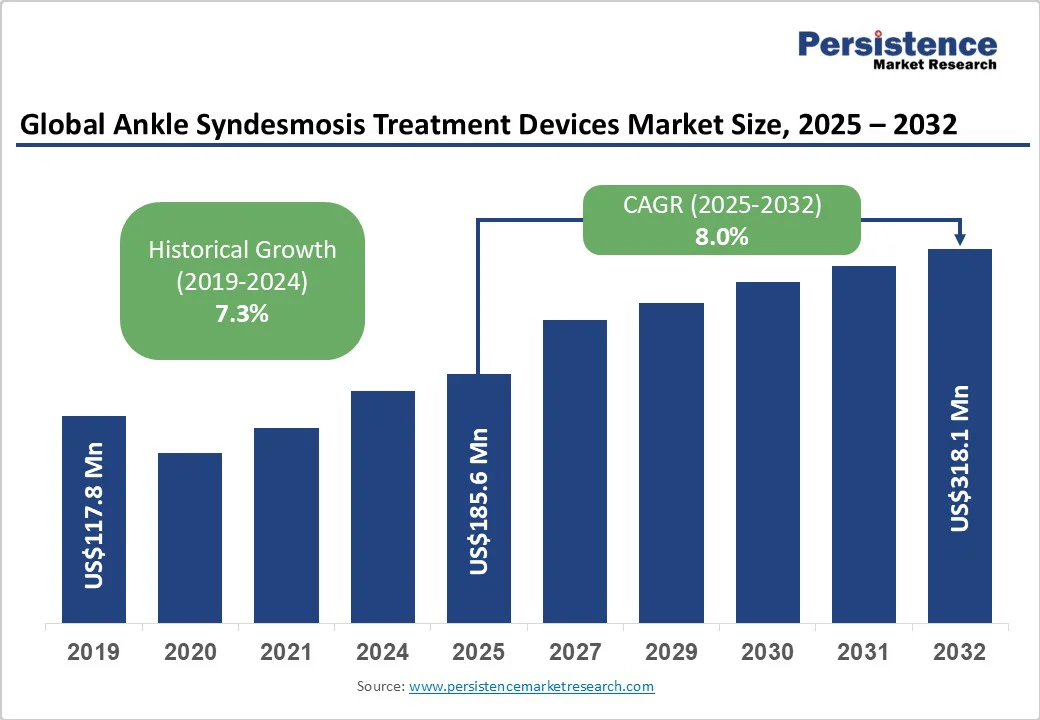
The global ankle syndesmosis treatment devices market size is likely to be valued at US$185.6 Million in 2025 and is estimated to reach US$318.1 Million in 2032, growing at a CAGR of 8.0% during the forecast period 2025 - 2032, driven by the rising incidence of sports-related injuries and an aging population prone to musculoskeletal fractures.
| Key Insights | Details |
|---|---|
| Ankle Syndesmosis Treatment Devices Market Size (2025E) | US$185.6 Mn |
| Market Value Forecast (2032F) | US$318.1 Mn |
| Projected Growth (CAGR 2025 to 2032) | 8.0% |
| Historical Market Growth (CAGR 2019 to 2024) | 7.3% |
The surging participation in competitive and recreational sports worldwide has led to a high incidence of ankle and lower-limb injuries, particularly among athletes. Activities such as football, basketball, and trail running frequently cause syndesmotic disruptions due to sudden twists, high-impact landings, or collisions. This surge in sports-related trauma has created a substantial demand for novel fixation devices that ensure precise anatomical repair while allowing early mobilization.
For instance, devices such as the Arthrex TightRope XP and Paragon 28 R3FLEX are mainly preferred in sports medicine clinics and hospital trauma units. These systems are preferred for their ability to provide dynamic stabilization, reduce post-operative complications, and expedite rehabilitation timelines. The continued expansion of youth and professional sports programs across North America, Europe, and Asia Pacific is expected to fuel the adoption of unique syndesmosis treatment devices.
Elderly populations are specifically susceptible to ankle fractures and syndesmotic injuries due to age-related declines in bone density, ligament elasticity, and balance. Falls and minor traumas, which might be negligible for young individuals, can lead to complex ankle injuries in older adults. This demographic trend has pushed healthcare providers to adopt novel treatment devices that deliver stable fixation and promote early weight-bearing, minimizing complications such as delayed healing or post-traumatic arthritis.
For example, the Acumed Acu-Sinch Knotless Implant is increasingly used in geriatric trauma centers, providing dynamic stabilization while reducing the requirement for secondary surgeries. As populations in Europe, North America, and parts of Asia Pacific continue to age, the demand for specialized syndesmosis treatment devices customized for elderly patients is projected to rise steadily.
One significant restraint on the growth of ankle syndesmosis treatment devices is the potential for hardware-related complications such as screw loosening, breakage, or migration. Traditional rigid screws can fail under stress, particularly in high-activity patients, leading to prolonged recovery, revision surgeries, and increased healthcare costs.
Even novel systems, if not properly implanted or maintained, tend to experience mechanical issues, which can undermine physician confidence and slow adoption in certain clinical settings. For example, reports of screw fatigue and occasional breakage in conventional syndesmosis fixation have prompted manufacturers such as Zimmer Biomet and Smith & Nephew to develop flexible and bioabsorbable alternatives designed to reduce hardware-related failures.
Accurate reduction of the syndesmotic joint is important for restoring ankle stability, and malreduction is a key challenge restraining market growth. Even minor misalignment during fixation can result in chronic pain, impaired mobility, and post-traumatic arthritis, thereby discouraging some surgeons from using new implant systems.
Devices such as suture button constructs and adjustable plate kits attempt to address this issue, but achieving precise anatomical alignment still requires high surgical expertise. Recent studies have shown that malreduction rates in conventional screw fixation can reach up to 20%, prompting the development of novel implants with real-time tension control and intraoperative imaging assistance.
The increasing preference for suture-button devices over traditional screws presents a significant growth opportunity in the global market. Suture-button systems provide dynamic stabilization that mimics the natural movement of the tibiofibular joint, reducing complications associated with rigid screws, such as breakage or the requirement for removal surgery.
These devices also allow early weight-bearing, fast rehabilitation, and improved functional outcomes, making them attractive in sports medicine and trauma care. Hospitals and specialized orthopedic clinics are now adopting suture-button systems due to their patient-centric benefits, creating opportunities for companies to expand their product lines.
Advancements in arthroscopic techniques and nanotechnology provide another key growth avenue for ankle syndesmosis treatment devices. Minimally invasive arthroscopic approaches allow surgeons to achieve precise syndesmotic reduction with small incisions, reducing tissue trauma and post-operative recovery time.
Nanotechnology is being explored to develop bioactive and biodegradable implants that improve bone healing, reduce infection risk, and improve fixation strength. For example, research into bioresorbable nanocomposite screws and suture coatings is enabling better integration with bone and soft tissue, improving long-term outcomes in trauma and geriatric patients.
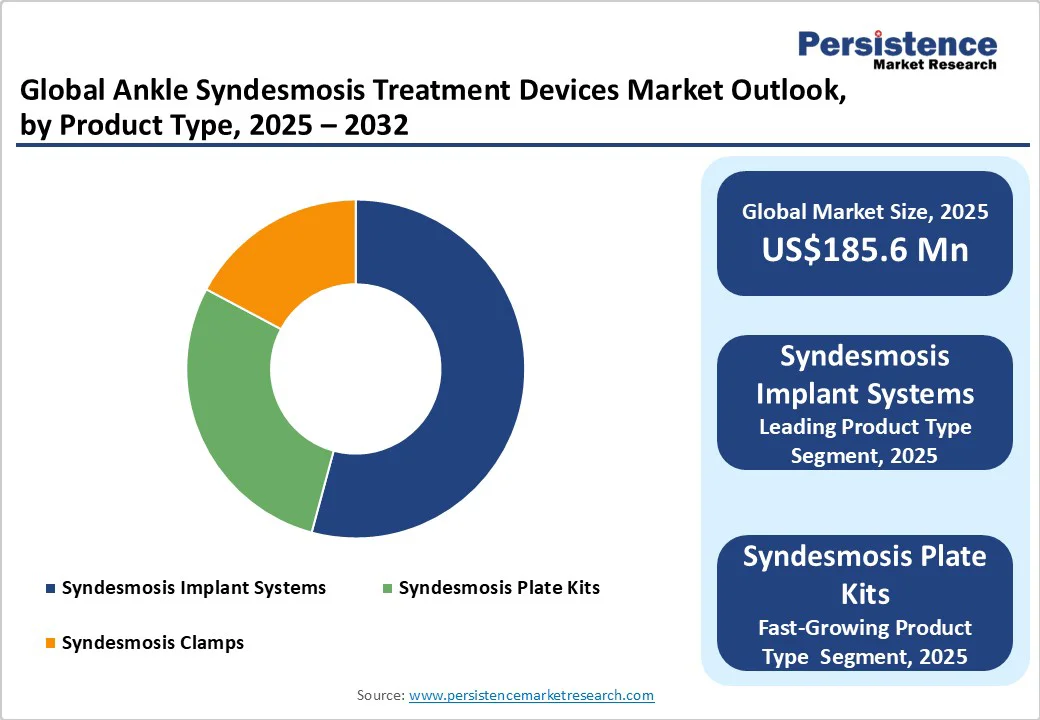
Syndesmosis implant systems are poised to hold a share of around 54.2% in 2025, owing to their ability to provide flexible fixation that mirrors the natural movement of the tibiofibular joint. This flexibility supports early weight-bearing and reduces the requirement for implant removal, which is often necessary with rigid screw fixations. Clinical studies have demonstrated that these systems deliver superior reduction, maintenance, and lower complication rates compared to traditional screws.
Syndesmosis plate kits are expected to see steady growth in the foreseeable future due to their ability to combine the benefits of screw fixation with the flexibility of a suture. This hybrid approach allows for precise, anatomical syndesmotic fixation while addressing limitations of traditional suture button constructs, including lack of tension control and medial soft-tissue disruption.
Ankle fractures are estimated to account for nearly 56.8% of the market share in 2025, as they often involve disruption of the tibiofibular ligaments, which are essential for ankle stability. Proper fixation is required to restore joint congruency and prevent long-term complications such as post-traumatic arthritis. Devices such as Arthrex’s TightRope are designed to provide stable fixation even in complex fracture patterns, allowing for early mobilization and quick functional recovery.
Syndesmosis reduction is important as accurate alignment of the tibiofibular joint determines long-term ankle function and prevents malreduction, which can lead to chronic pain and instability. Novel systems, including Acumed’s Acu-Sinch Knotless Implant, allow surgeons to achieve precise anatomical reduction with real-time tensioning control. These devices are valuable in high-energy injuries and sports-related trauma, where conventional screw fixation may not provide optimal joint congruity.
Hospitals are anticipated to hold a share of about 55.9% in 2025, as they handle high volumes of trauma and sports-related injuries, requiring immediate access to a range of fixation solutions. Hospitals also have the infrastructure for complex surgical procedures, including operating theaters, imaging facilities, and post-operative care units. These are essential for the successful implementation of advanced implants such as Arthrex’s TightRope.
Specialized orthopedic clinics are significant end users as they focus on targeted musculoskeletal care, allowing surgeons to adopt the latest minimally invasive and patient-specific fixation technologies. Clinics such as those in Germany and the U.S. integrate devices such as Acumed’s Acu-Sinch Knotless Implant into their treatment protocols, emphasizing personalized rehabilitation and fast return-to-activity timelines.
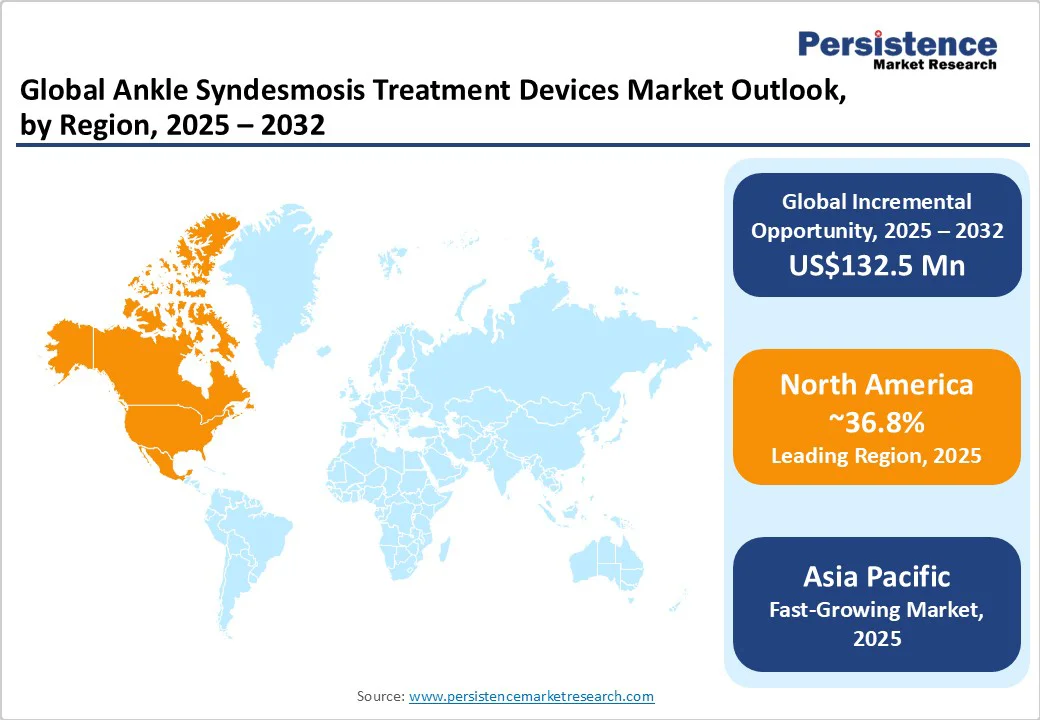
North America is expected to account for approximately 36.8% of the market share in 2025, owing to advanced healthcare infrastructure, high prevalence of sports-related injuries, and a robust pipeline of innovative solutions. The U.S. is predicted to hold a share of around 87.2% in 2032, underscoring its central role in the region's orthopedic landscape.
A notable trend in North America is the shift toward dynamic and adjustable fixation systems. For instance, Paragon 28's R3FLEX Stabilization System delivers surgeons the ability to precisely adjust and visualize tension during repair, aiming to mitigate complications such as post-traumatic arthritis. Similarly, Stryker's Gravity SynchFix device features a low-profile titanium design with ForceFiber suture, refining secure fixation while minimizing soft-tissue impingement.
In the forecast period, Asia Pacific will likely register a CAGR of nearly 7.7%, backed by increasing healthcare investments, rising sports participation, and a focus on novel medical technologies. China, Japan, and India are at the forefront, with China projected to hold a significant market share of approximately 40.6% in 2025. A notable development in Asia Pacific is Smith+Nephew's introduction of the INVISIKNOT Ankle Syndesmosis Repair Kit in Australia and New Zealand.
The low-profile and flexible device is designed to provide reliable fixation during the healing period following ankle syndesmotic trauma. Additionally, Acumed's Acu-Sinch Knotless Implant, launched in Asia Pacific, enables dynamic stabilization of the tibiofibular joint, providing a patient-specific solution for syndesmotic injuries.
Europe’s market is distinguished by strong collaboration between medical device companies and orthopedic research institutions. For example, Germany’s AO Foundation actively develops next-generation fixation systems, emphasizing biomechanical precision and anatomical restoration, which is shaping surgical protocols across Europe. Regulatory support in the EU encourages swift adoption of CE-marked biodegradable implants, allowing devices such as Inion’s bioabsorbable suture buttons to gain traction without lengthy approval delays.
The market is now embracing digital surgery integration, such as 3D preoperative planning and intraoperative navigation, to improve fixation accuracy in complex syndesmotic injuries. Companies such as Medacta are piloting these technologies, combining device design with digital guidance tools. Private hospitals in Europe are leading the uptake of hybrid systems that combine screw and suture fixation for customized patient outcomes.
The global ankle syndesmosis treatment devices market is evolving steadily. Key players include established orthopedic companies such as Arthrex, Smith & Nephew, Zimmer Biomet, and Paragon 28, as well as specialized firms, including Mortise Medical and Inion Oy. These companies are focusing on innovation to maintain a competitive edge.
The ankle syndesmosis treatment devices market is characterized by strategic emphasis on innovation, cost leadership, and market expansion. Companies are investing in research and development to launch advanced fixation systems, including biodegradable implants and knotless suture button devices, which improve patient outcomes and reduce complications.
Cost leadership is achieved through efficient manufacturing processes and strategic partnerships, enabling competitive pricing. Market expansion is pursued by entering emerging areas and forming alliances with healthcare providers to broaden product reach.
The ankle syndesmosis treatment devices market is projected to reach US$185.6 Million in 2025.
The rising sports-related injuries and an aging population, increasing ankle fracture cases, are a few key market drivers.
The ankle syndesmosis treatment devices market is poised to witness a CAGR of 8.0% from 2025 to 2032.
Shift toward minimally invasive procedures and the launch of biodegradable implants are the key market opportunities.
Arthrex, Inc., Mortise Medical, and Paragon 28, Inc. are a few key market players.
| Report Attribute | Details |
|---|---|
| Historical Data/Actuals | 2019 - 2024 |
| Forecast Period | 2025 - 2032 |
| Market Analysis | Value: US$ Bn |
| Geographical Coverage |
|
| Segmental Coverage |
|
| Competitive Analysis |
|
| Report Highlights |
|
By Product Type
By Application
By End User
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author