- Executive Summary
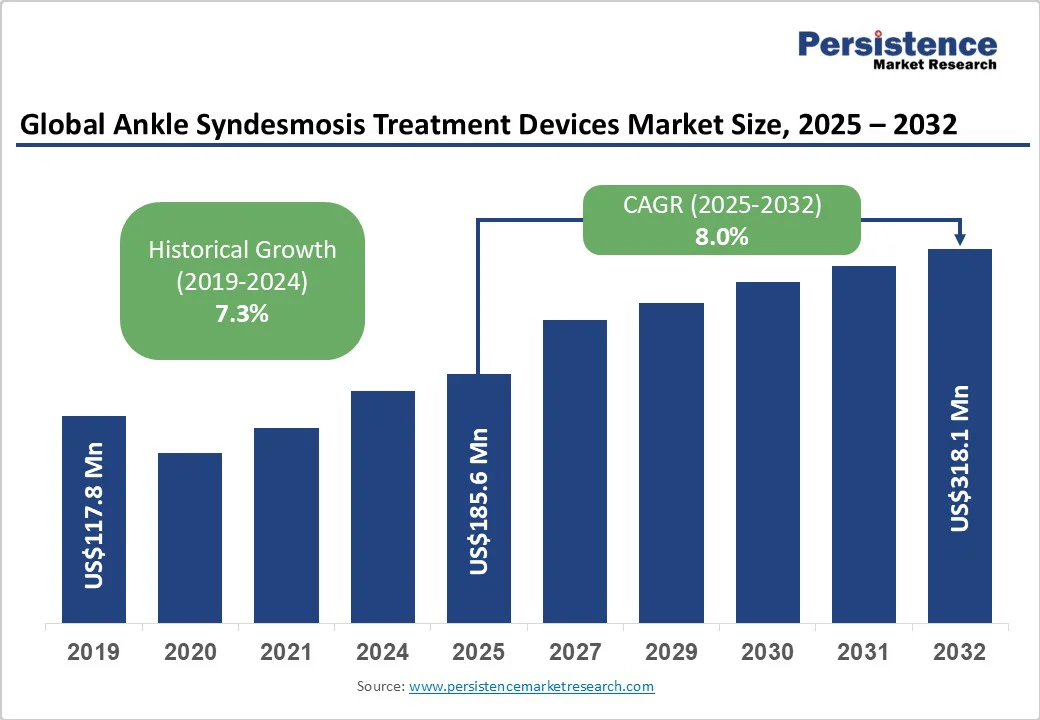
- Global Ankle Syndesmosis Treatment Devices Market Snapshot, 2025 and 2032
- Market Opportunity Assessment, 2025 - 2032, US$ Bn
- Key Market Trends
- Future Market Projections
- Premium Market Insights
- Industry Developments
- PMR Analysis and Recommendations
- Market Overview
- Market Scope and Definition
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Challenges
- Key Trends
- COVID-19 Impact Analysis
- Forecast Factors - Relevance and Impact
- Value Chain Analysis
- Supply Chain Analysis
- List of Key Market Players
- Value Added Insights
- PESTLE Analysis
- Porter’s Five Force Analysis
- Price Trend Analysis, 2019 - 2032
- Pricing Analysis, By Product Type
- Key Factors Impacting Prices, By Application
- Global Ankle Syndesmosis Treatment Devices Market Outlook
- Key Highlights
- Market Size (US$ Bn) and Y-o-Y Growth
- Absolute $ Opportunity
- Market Size (US$ Bn) Analysis and Forecast
- Historical Market Size (US$ Bn) Analysis, 2019-2024
- Market Size (US$ Bn) Analysis and Forecast, 2025-2032
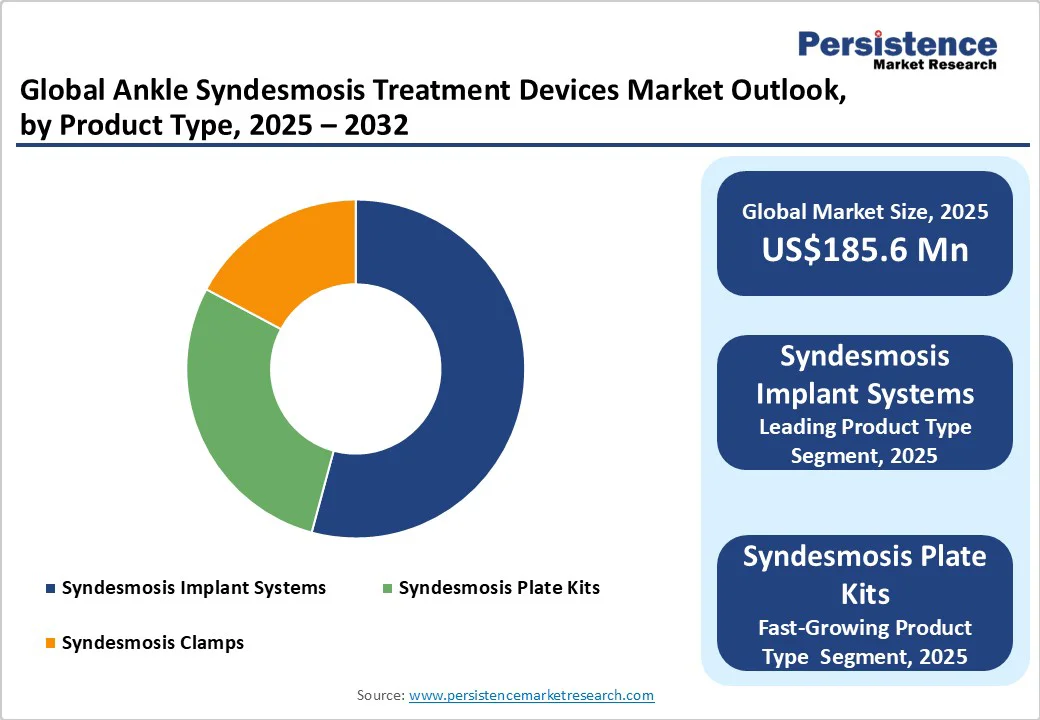
- Global Ankle Syndesmosis Treatment Devices Market Outlook: Product Type
- Historical Market Size (US$ Bn) Analysis, By Product Type, 2019-2024
- Market Size (US$ Bn) Analysis and Forecast, By Product Type, 2025-2032
- Syndesmosis Implant Systems
- Syndesmosis Plate Kits
- Syndesmosis Clamps
- Market Attractiveness Analysis: Product Type
- Global Ankle Syndesmosis Treatment Devices Market Outlook: Application
- Historical Market Size (US$ Bn) Analysis, By Application, 2019-2024
- Market Size (US$ Bn) Analysis and Forecast, By Application, 2025-2032
- Ankle Fractures
- Syndesmosis Reduction
- Postoperative Management
- Market Attractiveness Analysis: Application
- Global Ankle Syndesmosis Treatment Devices Market Outlook: End-user
- Historical Market Size (US$ Bn) Analysis, By End-user, 2019-2024
- Market Size (US$ Bn) Analysis and Forecast, By End-user, 2025-2032
- Hospitals
- Ambulatory Settings
- Specialized Orthopedic Clinics
- Market Attractiveness Analysis: End-user
- Key Highlights
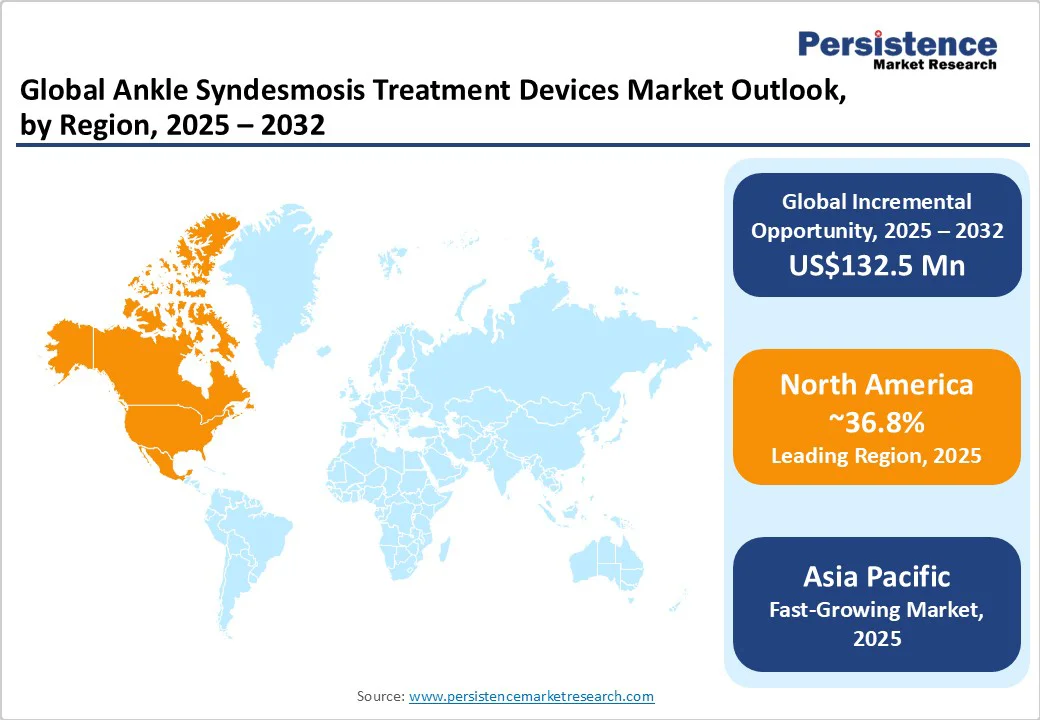
- Global Ankle Syndesmosis Treatment Devices Market Outlook: Region
- Historical Market Size (US$ Bn) Analysis, By Region, 2019-2024
- Market Size (US$ Bn) Analysis and Forecast, By Region, 2025-2032
- North America
- Europe
- East Asia
- South Asia and Oceania
- Latin America
- Middle East & Africa
- Market Attractiveness Analysis: Region
- North America Ankle Syndesmosis Treatment Devices Market Outlook
- Historical Market Size (US$ Bn) Analysis, By Market, 2019-2024
- By Country
- By Product Type
- By Application
- By End-user
- Market Size (US$ Bn) Analysis and Forecast, By Country, 2025-2032
- U.S.
- Canada
- Market Size (US$ Bn) Analysis and Forecast, By Product Type, 2025-2032
- Syndesmosis Implant Systems
- Syndesmosis Plate Kits
- Syndesmosis Clamps
- Market Size (US$ Bn) Analysis and Forecast, By Application, 2025-2032
- Ankle Fractures
- Syndesmosis Reduction
- Postoperative Management
- Market Size (US$ Bn) Analysis and Forecast, By End-user, 2025-2032
- Hospitals
- Ambulatory Settings
- Specialized Orthopedic Clinics
- Market Attractiveness Analysis
- Historical Market Size (US$ Bn) Analysis, By Market, 2019-2024
- Europe Ankle Syndesmosis Treatment Devices Market Outlook
- Historical Market Size (US$ Bn) Analysis, By Market, 2019-2024
- By Country
- By Product Type
- By Application
- By End-user
- Market Size (US$ Bn) Analysis and Forecast, By Country, 2025-2032
- Germany
- France
- U.K.
- Italy
- Spain
- Russia
- Türkiye
- Rest of Europe
- Market Size (US$ Bn) Analysis and Forecast, By Product Type, 2025-2032
- Syndesmosis Implant Systems
- Syndesmosis Plate Kits
- Syndesmosis Clamps
- Market Size (US$ Bn) Analysis and Forecast, By Application, 2025-2032
- Ankle Fractures
- Syndesmosis Reduction
- Postoperative Management
- Market Size (US$ Bn) Analysis and Forecast, By End-user, 2025-2032
- Hospitals
- Ambulatory Settings
- Specialized Orthopedic Clinics
- Market Attractiveness Analysis
- Historical Market Size (US$ Bn) Analysis, By Market, 2019-2024
- East Asia Ankle Syndesmosis Treatment Devices Market Outlook
- Historical Market Size (US$ Bn) Analysis, By Market, 2019-2024
- By Country
- By Product Type
- By Application
- By End-user
- Market Size (US$ Bn) Analysis and Forecast, By Country, 2025-2032
- China
- Japan
- South Korea
- Market Size (US$ Bn) Analysis and Forecast, By Product Type, 2025-2032
- Syndesmosis Implant Systems
- Syndesmosis Plate Kits
- Syndesmosis Clamps
- Market Size (US$ Bn) Analysis and Forecast, By Application, 2025-2032
- Ankle Fractures
- Syndesmosis Reduction
- Postoperative Management
- Market Size (US$ Bn) Analysis and Forecast, By End-user, 2025-2032
- Hospitals
- Ambulatory Settings
- Specialized Orthopedic Clinics
- Market Attractiveness Analysis
- Historical Market Size (US$ Bn) Analysis, By Market, 2019-2024
- South Asia & Oceania Ankle Syndesmosis Treatment Devices Market Outlook
- Historical Market Size (US$ Bn) Analysis, By Market, 2019-2024
- By Country
- By Product Type
- By Application
- By End-user
- Market Size (US$ Bn) Analysis and Forecast, By Country, 2025-2032
- India
- Southeast Asia
- ANZ
- Rest of South Asia & Oceania
- Market Size (US$ Bn) Analysis and Forecast, By Product Type, 2025-2032
- Syndesmosis Implant Systems
- Syndesmosis Plate Kits
- Syndesmosis Clamps
- Market Size (US$ Bn) Analysis and Forecast, By Application, 2025-2032
- Ankle Fractures
- Syndesmosis Reduction
- Postoperative Management
- Market Size (US$ Bn) Analysis and Forecast, By End-user, 2025-2032
- Hospitals
- Ambulatory Settings
- Specialized Orthopedic Clinics
- Market Attractiveness Analysis
- Historical Market Size (US$ Bn) Analysis, By Market, 2019-2024
- Latin America Ankle Syndesmosis Treatment Devices Market Outlook
- Historical Market Size (US$ Bn) Analysis, By Market, 2019-2024
- By Country
- By Product Type
- By Application
- By End-user
- Market Size (US$ Bn) Analysis and Forecast, By Country, 2025-2032
- Brazil
- Mexico
- Rest of Latin America
- Market Size (US$ Bn) Analysis and Forecast, By Product Type, 2025-2032
- Syndesmosis Implant Systems
- Syndesmosis Plate Kits
- Syndesmosis Clamps
- Market Size (US$ Bn) Analysis and Forecast, By Application, 2025-2032
- Ankle Fractures
- Syndesmosis Reduction
- Postoperative Management
- Market Size (US$ Bn) Analysis and Forecast, By End-user, 2025-2032
- Hospitals
- Ambulatory Settings
- Specialized Orthopedic Clinics
- Market Attractiveness Analysis
- Historical Market Size (US$ Bn) Analysis, By Market, 2019-2024
- Middle East & Africa Ankle Syndesmosis Treatment Devices Market Outlook
- Historical Market Size (US$ Bn) Analysis, By Market, 2019-2024
- By Country
- By Product Type
- By Application
- By End-user
- Market Size (US$ Bn) Analysis and Forecast, By Country, 2025-2032
- GCC Countries
- Egypt
- South Africa
- Northern Africa
- Rest of Middle East & Africa
- Market Size (US$ Bn) Analysis and Forecast, By Product Type, 2025-2032
- Syndesmosis Implant Systems
- Syndesmosis Plate Kits
- Syndesmosis Clamps
- Market Size (US$ Bn) Analysis and Forecast, By Application, 2025-2032
- Ankle Fractures
- Syndesmosis Reduction
- Postoperative Management
- Market Size (US$ Bn) Analysis and Forecast, By End-user, 2025-2032
- Hospitals
- Ambulatory Settings
- Specialized Orthopedic Clinics
- Market Attractiveness Analysis
- Historical Market Size (US$ Bn) Analysis, By Market, 2019-2024
- Competition Landscape
- Market Share Analysis, 2025
- Market Structure
- Competition Intensity Mapping By Market
- Competition Dashboard
- Company Profiles (Details - Overview, Financials, Strategy, Recent Developments)
- Arthrex, Inc.
- Overview
- Segments and Product Type
- Key Financials
- Market Developments
- Market Strategy
- Mortise Medical
- Paragon 28, Inc.
- Inion Oy
- Smith & Nephew Plc.
- Breg
- Wright Medical Group N.V.
- Acumed LLC
- Exatech, Inc.
- Zimmer Biomet Holdings, Inc.
- Infinite Technologies
- Arthrex, Inc.
- Appendix
- Research Methodology
- Research Assumptions
- Acronyms and Abbreviations

Loading page data
Please wait a moment