ID: PMRREP3193| 180 Pages | 29 Apr 2025 | Format: PDF, Excel, PPT* | Healthcare

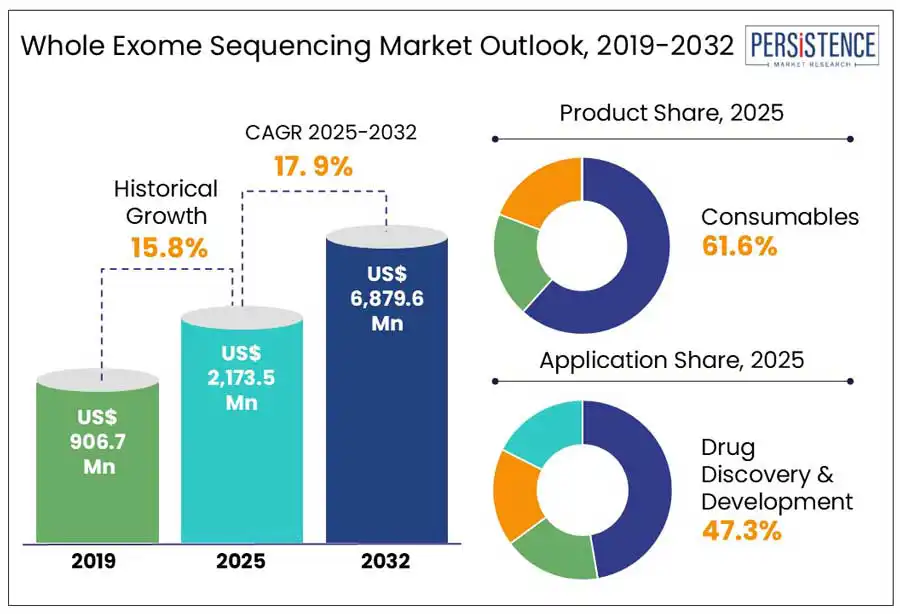
According to the Persistence Market Research, the global whole exome sequencing market size is estimated to grow from US$ 2,173.5 Mn in 2025 to US$ 6,879.6 Mn by 2032 and record a CAGR of 17.9% by 2032. Increasing adoption of precision medicine, growing integration of WES into clinical practice, and collaboration between genomics companies and healthcare are the key drivers. Expanding insurance coverage for WES in clinical and diagnostic applications and a surge in domestic manufacturers aimed to reduce the overall cost and accessibility of sequencing technology are further accelerating market expansion.
Key Highlights
|
Global Market Attribute |
Key Insights |
|
Whole Exome Sequencing Market Size (2025E) |
US$ 2,173.5 Mn |
|
Market Value Forecast (2032F) |
US$ 6,879.6 Mn |
|
Projected Growth (CAGR 2025 to 2032) |
17.9% |
|
Historical Market Growth (CAGR 2019 to 2024) |
15.8% |
The growing integration of WES into clinical practice is transforming the overall landscape of clinical diagnostics and precision medicine. Population genomic initiatives such as National Institutes of Health’s All of Us Research Program, are driving the global market by providing massive genomic datasets linked to clinical data. Launched in 2019, the data now includes over a quarter million whole genome sequences from over 413,450 individuals, all available for research use.
The database further includes information related to electronic health records (EHR), health surveys, biospecimens and physical measurements among others. Such comprehensive data collection enables researchers to explore complex gene-environment interactions, enhance diagnostic accuracy, validate the utility of WES at scale, and understand their impact on health outcomes. The establishment of high-quality clinical-genomic feedback loops is anticipated to propel the global whole exome sequencing market forward.
Whole exome sequencing offers significant advantages over single gene or panel tests and whole genome sequencing (WGS) tests in terms of obtaining genetic information and cost-efficiency. It provides insightful data for patients with complex diseases of uncertain or heterogeneous genetic origin, thus increasing accessibility to clinical testing.
However, WES has its limitations. The current WES technology does not cover 100% of the exome, leaving certain exons unexplored and potentially missing disease-causing variants. Additionally, WES has limited sensitivity in detecting structural variations (SVs), with WGS and chromosomal microarrays (CMA) typically offering more accurate identification of SVs.
Moreover, WES does not include sequencing of some of the non-coding intron regions such as enhancers and long-noncoding RNAs. A meta-analysis comparing WES, WGS, and CMA in diagnosing genetic disorders in children revealed a higher diagnostic yield of WGS (41%) compared to WES (36%) highlighting, while WES is effective, they may not be suitable for complex or uncertain genetic disorders. Such limitations highlight the need for additional testing to capture missed regions, confirm detected variants, or detect elusive variants. Addressing these gaps is expected to drive further advancements and accelerate the growth of the global whole exome sequencing market.
The growing collaboration between genomic companies and healthcare providers presents a significant opportunity in the global whole exome sequencing market. Genome Medical collaborated with Nest Genomics in January 2025 to expand access to genetic services, offering timely test ordering, medical consultations, genetic counselling, and personalized treatment.
In April 2024, SOPHiA GENETICS joined forces with Strand Life Sciences to advance precision medicine initiatives and improve the use of genomic data to enhance patient care. Flatiron Health collaborated with Caris Life Sciences in January 2024 to integrate clinical data from Flatiron’s EHR information with genomic insights from Caris’ DNA, RNA, and imaging data, advancing oncology research.
These partnerships facilitate seamless integration of WES into clinical practice, improving diagnosis and treatment plans, particularly for oncology and rare diseases. As healthcare systems embrace precision medicine, such collaborations are poised to drive the widespread adoption and growth of WES in clinical settings.
Consumables segment is projected to account for a revenue share of 61.6% in 2025 within the global market. The growing adoption of WES for research, clinical diagnostics and personalized medicine fuels the demand for consumables such as kits, library preparation reagents, sequencing plates, and capture probes.
In comparison to instruments and services, consumables are in significantly high demand due to its repeated use in every sequencing run, making them a continuous revenue source. This consistent demand is projected to ensure that consumables dominate the revenue share in the global WES market.
Drug discovery & development segment is expected to dominate the application category in 2025 with 47.3% market share. The segment’s dominance is driven by increasing use of WES to identify a broader spectrum of genetic variations, understand disease pathways, and accelerate biomarker discovery. These are the critical components in the drug development process.
Unlike clinical diagnostics, drug discovery demands large-scale genomic analysis across diverse populations. Several pharma and biotech companies invest heavily in WES research and development, alongside government-funded initiatives such as China’s Metabolic Analytics Project (ChinaMAP) and Germany’s National Network for Genomic Medicine (nNGM). Growing demand for precision therapies is expected to drive the integration of WES into drug discovery advancing innovative treatments and expanding the global market.
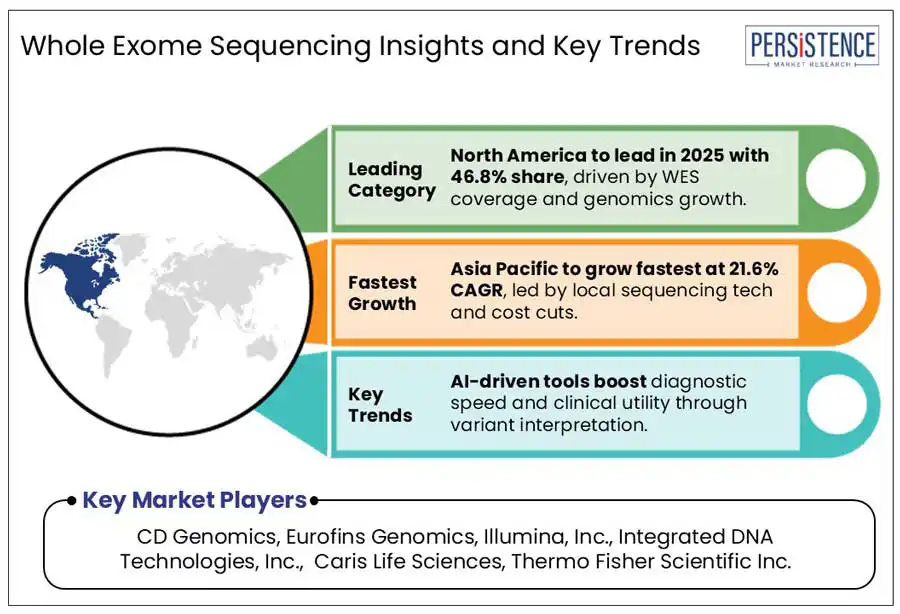
North America is anticipated to hold 46.8% of the global market share in 2025, driven by growing insurance coverage for WES, large-scale genomics programs and FDA-backed diagnostic integration driving the clinical adoption.
Whole genome sequencing (WGS) and exome sequencing are widely used to diagnose suspected genetic disorders. The U.S. has been a frontrunner in integrating WES into insurance coverage, particularly for oncology, rare genetic disorders in children, and neurodevelopmental conditions.
A recent study revealed an increase in the payer coverage for both WGS and WES across the region. Currently, half of insurance population have coverage for only WES, 12% have coverage for both WGS & WES, while 37% have no coverage for either. This growing insurance coverage is expected to boost WES adoption in clinical settings, further expanding the U.S. whole exome sequencing market.
Europe is estimated to account for a market share of 25.2% in 2025. Robust national genomics programs and government-backed healthcare reforms are a few driving factors for market growth across European countries such as Germany and the U.K.
Strong healthcare policies and government-led initiatives actively steer the Germany whole exome sequencing market. Initiatives like National Network Genomic Medicine (nNGM) and German National Genome Network support the genomic research activity and clinical integration of WES.
The nNGM offers next-generation sequencing (NGS)-based diagnostic testing and recommendation for off-label use and clinical trial participation to 23 centers in 27 locations. In 2024, nearly 500 healthcare facilities including both hospitals and private clinics performed centralized NGS-based molecular multiplex diagnostics for patients with advanced lung cancer in Germany. These initiatives underscore Germany's commitment to incorporating WES within routine healthcare and medical research workflows.
The industry in Asia Pacific is estimated to grow by 21.6% during the forecast period. The market is driven by unique regional dynamics, rapid advancement in genomic infrastructure and the rising number of domestic manufacturers for sequencing technologies and consumables across Asian countries such as India and China.
Increasing prevalence of rare genetic disorders and rising demand for affordable diagnostic solutions are driving the need for national initiatives such as IndiGen initiative by CSIR in India. The IndiGen programme aims to undertake whole genome sequencing of thousands of Indian individuals to enable genetic epidemiology and develop public health technologies applications using genomic data. The push towards “Make in India” is further encouraging domestic manufacturing of genomic equipment, bringing down the cost of WES-based testing.
Furthermore, the launch of the Framework for Exchange of Data Protocols (FeED) and the Indian Biological Data Centre (IBDC) Portals in January 2025 is expected to provide global access to 10,000 whole genome samples, decreasing India’s dependency on foreign genomic data. This growing infrastructure and strong government support are set to drive the expansion of India whole exome sequencing market.
China, on the other hand, has also developed significant genomic infrastructure from initiatives such as National Genomics Data Center (NGDC) and China Metabolic Analytics Project (ChinaMAP) driven by private sector investments and govt. initiatives. Chinese companies such as BGI and Berry Genomics are also playing a key role in localizing sequencing technology and reducing costs, further increasing regional accessibility. This has led to widespread adoption of WES in standard diagnostic protocols, driving the China whole exome sequencing market.
The global whole exome sequencing market is moderately fragmented with a diverse range of participants. Both global and domestic players offer innovative technologies and solutions driving market competition. Collaborations and partnerships are the key strategies adopted within this landscape, along with government-funded genomic initiatives, further intensifying the competition.
The global market is set to reach US$ 2,173.5 Mn in 2025.
The market is projected to record a CAGR of 17.9% during the forecast period from 2025 to 2032.
Increasing prevalence of rare genetic disorders and the growing demand for affordable diagnostic solution is expected to drive the global market.
CD Genomics, Eurofins Genomics, Illumina, Inc., Integrated DNA Technologies, Inc., Caris Life Sciences, Thermo Fisher Scientific Inc., and Laboratory Corporation of America® Holdings are a few leading players.
North America is projected to dominate the global market in 2025.
|
Report Attributes |
Details |
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis Units |
Value: US$ Mn/Bn Volume: Units |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
|
Customization and Pricing |
Available upon request |
By Product:
By Application:
By Technology:
By End-User:
By Region:
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author