ID: PMRREP31450| 170 Pages | 22 Oct 2025 | Format: PDF, Excel, PPT* | Healthcare

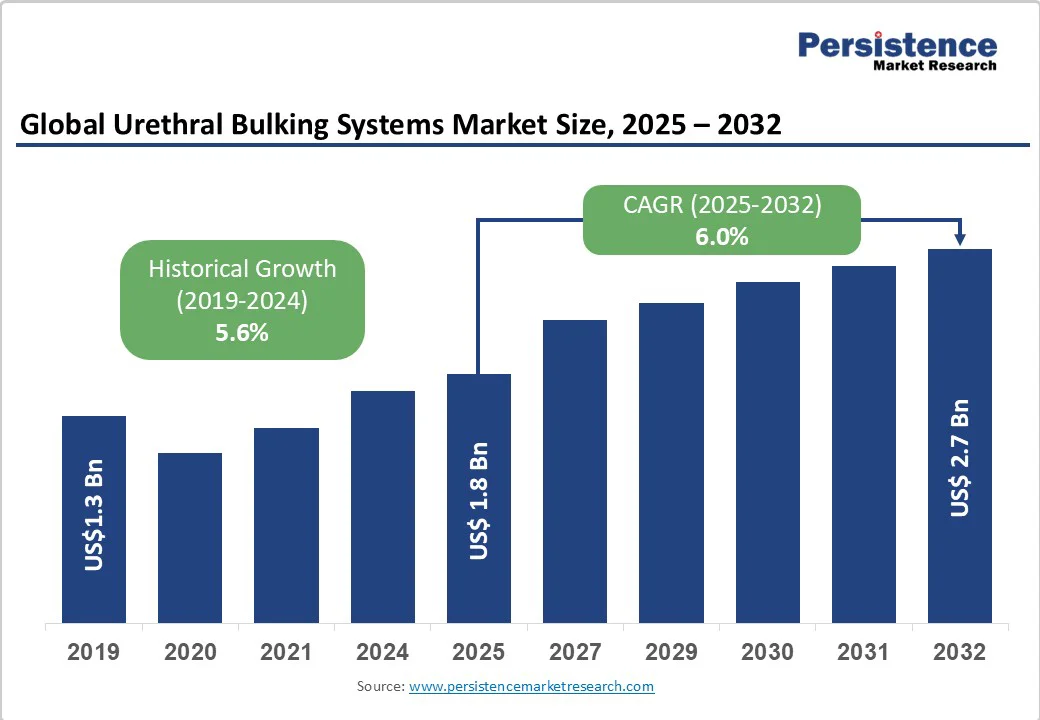
The global urethral bulking systems market size is likely to be valued at US$1.8 Billion in 2025 and is estimated to reach US$2.7 Billion in 2032, growing at a CAGR of 6.0% during the forecast period 2025 - 2032, driven by the rising prevalence of stress urinary incontinence, especially among aging populations and women affected by childbirth-related pelvic floor weakening.
| Key Insights | Details |
|---|---|
| Urethral Bulking Systems Market Size (2025E) | US$1.8 Billion |
| Market Value Forecast (2032F) | US$2.7 Billion |
| Projected Growth (CAGR 2025 to 2032) | 6.0% |
| Historical Market Growth (CAGR 2019 to 2024) | 5.6% |
An increasing number of women are being diagnosed with Stress Urinary Incontinence (SUI), primarily due to factors such as childbirth, aging, obesity, and pelvic floor damage. Studies show that nearly one in three women globally experience some form of urinary leakage during their lifetime, with prevalence rising sharply after menopause.
This surging patient pool has strengthened the clinical requirement for urethral bulking systems as a conservative yet effective treatment alternative for mild to moderate SUI. For instance, the 2024 International Urogynecology Journal reported a notable rise in outpatient bulking procedures in Europe, specifically among elderly women seeking low-risk solutions over invasive sling surgeries.
There is an increasing clinical preference for minimally invasive options that reduce hospitalization time and recovery risks. Urethral bulking systems meet this demand, providing same-day outpatient procedures with minimal anesthesia and a quick return to normal activity.
Recent hospital data from the U.K. National Health Service (NHS) in 2024 indicated that day-case bulking treatments saw a 30% year-on-year rise, mainly due to improved injection systems and fewer postoperative complications. This convenience and reduced surgical burden have made bulking a preferred first-line or secondary treatment for patients, avoiding complex reconstructive surgeries.
While urethral bulking systems provide a minimally invasive alternative to slings, their long-term efficacy often falls short. Several patients experience symptom recurrence within a few years, requiring repeat procedures to maintain continence.
Clinical data published in the International Neurourology Journal (2024) indicated that success rates for bulking agents decline significantly after three to five years, especially in women with severe stress urinary incontinence. In contrast, mid-urethral slings tend to provide more durable outcomes, making bulking agents a less preferred option for long-term management.
The effectiveness of bulking procedures can vary widely depending on the injection technique, the material used, and patient anatomy. Some individuals achieve only partial improvement, necessitating additional treatments.
A 2024 multicenter study from Sweden found that nearly 40% of patients required a second bulking session within two years to sustain continence. This variability in therapeutic response and the requirement for repeat interventions can discourage patients and clinicians from choosing bulking systems as a primary solution.
Research is moving toward next-generation bulking materials that promote better tissue integration and long-lasting results. Bioceramic-based agents are being designed to trigger a mild, controlled inflammatory response that improves collagen formation around the urethra, leading to more stable outcomes.
In 2024, a team at the University of Zurich developed a calcium-phosphate bioceramic hydrogel that showed superior biocompatibility and minimal migration risk in animal models. Such developments aim to overcome the limitations of traditional silicone or polymer gels, providing high durability and fewer repeat injections.
Stem cell-based bulking approaches are being explored as a regenerative alternative to synthetic agents. Early clinical trials in Japan and South Korea have demonstrated that adipose-derived stem cell injections can restore urethral sphincter function by regenerating muscle tissue, rather than merely providing mechanical support.
A 2025 pilot study published in Stem Cells Translational Medicine reported improved continence rates and sustained sphincter contractility over 18 months. It signaled superior potential for stem cell-enhanced bulking systems to transform future treatment standards.
Injectable materials are anticipated to lead with a share of around 43.6% in 2025 as they allow quick, minimally invasive treatment with minimal downtime and discomfort.
Modern bulking agents such as polyacrylamide hydrogel (Bulkamid) and silicone-based Macroplastique are designed for easy tissue integration, reducing migration and allergic reactions. Their injectable nature makes them ideal for outpatient settings, where most procedures are completed in under 30 minutes without general anesthesia.
Delivery systems are estimated to witness a considerable CAGR through 2032. These are evolving to improve precision, safety, and ease of use during bulking procedures.
Manufacturers are developing ergonomically designed, single-use injectors and ultrasound-guided applicators that help clinicians place the bulking agent more accurately, minimizing leakage or overcorrection. Hospitals and clinics are adopting these novel delivery devices as they shorten procedure time and improve reproducibility across users.
Biocompatible materials are expected to capture a share of nearly 49.1% in 2025, as they minimize tissue irritation, allergic reactions, and migration.
New agents such as polyacrylamide hydrogel (Bulkamid) and calcium hydroxylapatite microspheres integrate smoothly into urethral tissue, promoting a stable bulking effect without causing inflammation. Clinical studies in the European Urology Journal (2024) showed that biocompatible hydrogels maintained over 90% structural integrity after two years, with no cases of foreign body granuloma formation.
Natural and bio-derived bulking substances are gaining attention for their regenerative and low-immunogenic properties.
Researchers are exploring collagen, hyaluronic acid, and alginate-based composites that mimic natural tissue and stimulate mild collagen synthesis for improved urethral closure. A 2025 study from the University of Tokyo demonstrated that hyaluronic acid-gelatin hydrogels achieved better tissue compatibility and faster healing compared to synthetic agents.
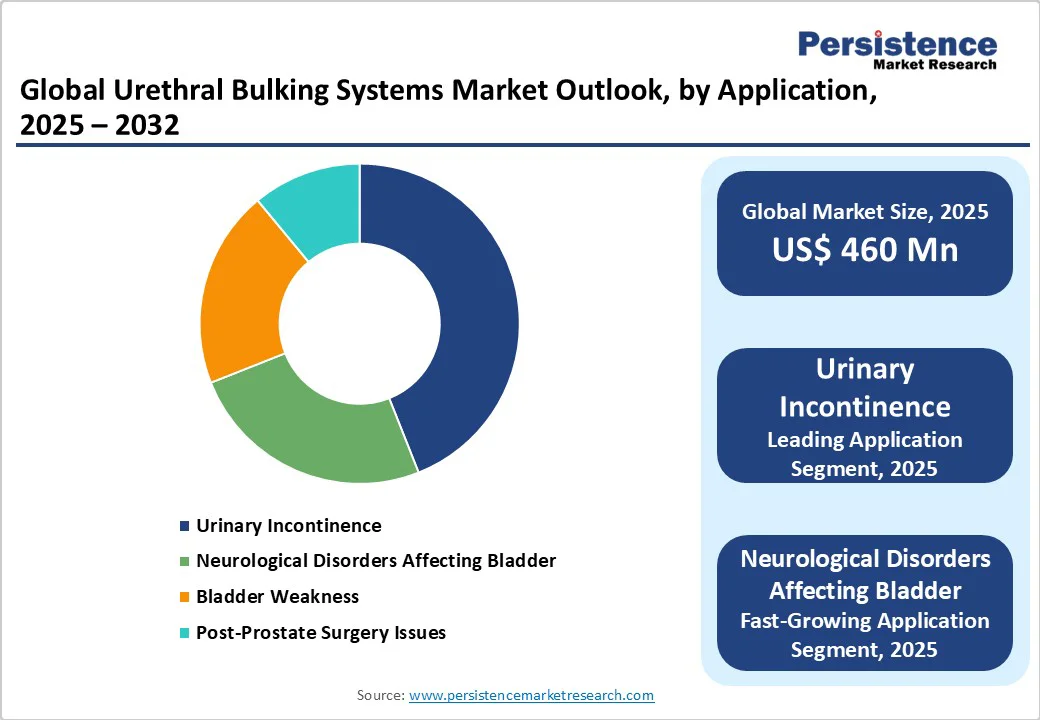
Stress Urinary Incontinence (SUI) is speculated to account for approximately 55.3% of the share in 2025, as it is the most common form of incontinence among women, primarily caused by childbirth, hormonal decline after menopause, and pelvic floor weakening.
Urethral bulking agents directly address this problem by increasing urethral resistance and restoring closure pressure without the requirement for surgery. According to a 2024 study in the American Journal of Obstetrics and Gynecology, nearly 50% of women over 40 experience some degree of SUI, making it the largest patient pool for bulking procedures.
Urinary sphincter deficiency, often resulting from nerve damage or tissue atrophy, is another prominent indication boosting the use of bulking systems. It weakens the urethral closure mechanism, leading to persistent leakage even in the absence of physical strain.
Bulking agents help restore sphincter coaptation by improving urethral support and resistance to urine flow. This indication is mainly relevant in post-prostatectomy male patients and elderly women with Intrinsic Sphincter Deficiency (ISD).
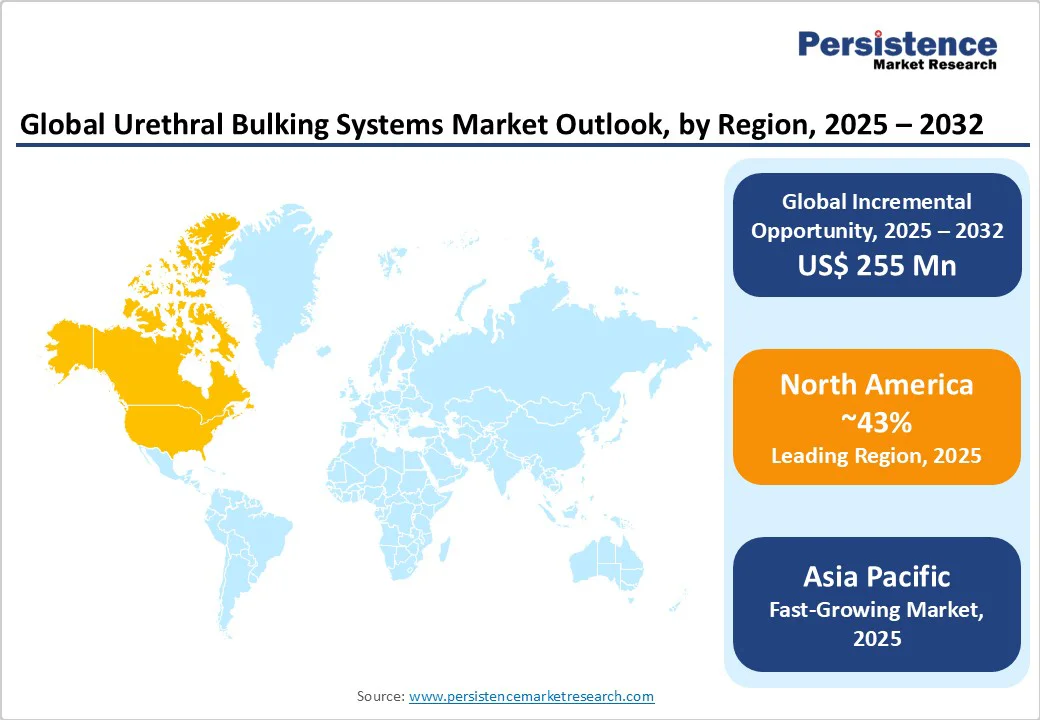
In 2025, North America is poised to hold nearly 33.8% of the market share, as urethral bulking systems are seeing steady adoption, especially among older women and patients seeking minimally invasive alternatives to slings. The trend has strengthened since the FDA approval of Bulkamid in 2020, which provided physicians with a safe and long-lasting hydrogel option.
Clinical studies in the U.S. between 2019 and 2024 showed that Bulkamid achieved more than 70% patient satisfaction within a year, pushing its preference in outpatient urology centers. The system’s ease of use and low complication rates have made it a popular choice for those unfit for surgery or unwilling to undergo invasive procedures. In Canada, usage has been slower due to variations in provincial reimbursement policies, but demand is rising as clinicians report improved long-term results with new materials.
In Europe, urethral bulking systems are gaining traction as effective treatments for SUI, specifically among older patients and those seeking minimally invasive options. Leading countries such as Germany, France, and the U.K. are augmenting this growth due to novel healthcare infrastructure and increasing awareness of urinary health issues. Pyrolytic carbon-coated graphite beads are the most used bulking agents in Europe, accounting for over half of the market share in 2024.
These materials are favored for their high durability and biocompatibility, which contribute to stable and long-lasting outcomes. However, the adoption of urethral bulking systems varies across countries in the region due to differences in healthcare policies and reimbursement structures. While some countries have integrated these treatments into their standard care protocols, others are still evaluating their cost-effectiveness and long-term benefits.
In Asia Pacific, urethral bulking systems are experiencing steady growth, propelled by an aging population, increasing prevalence of urinary incontinence, and developments in minimally invasive procedures. China, India, Japan, and Australia are at the forefront of this expansion, with rising disposable income and improving healthcare infrastructure facilitating greater access to these treatments.
The adoption of urethral bulking systems in Asia Pacific is further supported by the increasing number of urology clinics and ambulatory surgical centers, which provide these minimally invasive procedures. Technological developments in bulking agents, such as the emergence of biocompatible materials and improved delivery systems, have improved the efficacy and safety of these treatments, making them more appealing to both healthcare providers and patients.
The global urethral bulking systems market is concentrated, with only a few players holding significant market presence. Contura’s Bulkamid and Laborie’s Macroplastique are the two dominant products globally.
Bulkamid, a polyacrylamide hydrogel, has gained traction in the last few years owing to its minimally invasive nature and durable results, supported by several clinical studies showing over 80% patient satisfaction two years post-procedure. Laborie’s Macroplastique, which uses silicone particles, remains a trusted choice for long-term volume stability and is widely used across Europe and North America.
The urethral bulking systems market is projected to reach US$1.8 Billion in 2025.
Increasing preference for minimally invasive treatments and rising prevalence of SUI are the key market drivers.
The urethral bulking systems market is poised to witness a CAGR of 6.0% from 2025 to 2032.
The development of regenerative materials and the adoption of biocompatible bulking agents are the key market opportunities.
CR Bard, Merz Aesthetics, and Cogentix Medical are a few key market players.
| Report Attribute | Details |
|---|---|
| Historical Data/Actuals | 2019 - 2024 |
| Forecast Period | 2025 - 2032 |
| Market Analysis | Value: US$ Bn |
| Geographical Coverage |
|
| Segmental Coverage |
|
| Competitive Analysis |
|
| Report Highlights |
|
By Product Type
By Material
By Indication
By End-user
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author