ID: PMRREP4289| 240 Pages | 15 Jan 2026 | Format: PDF, Excel, PPT* | Healthcare

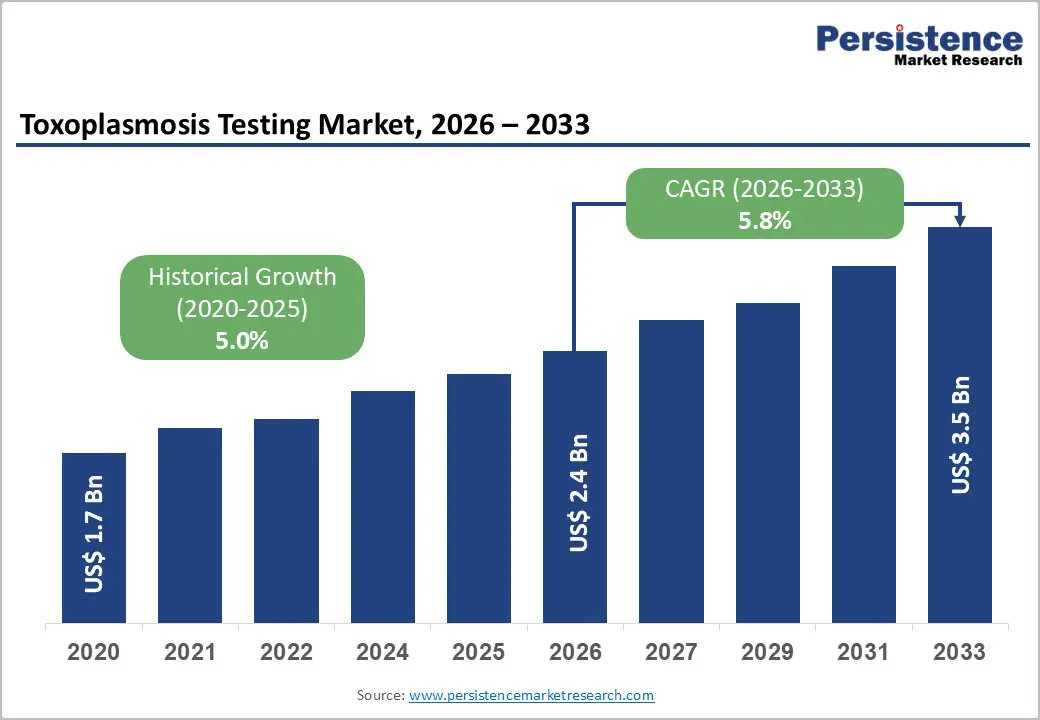
The global toxoplasmosis testing market is estimated to grow from US$ 2.4 Bn in 2026 to US$ 3.5 Bn by 2033. The market is projected to record a CAGR of 5.8% during the forecast period from 2026 to 2033.
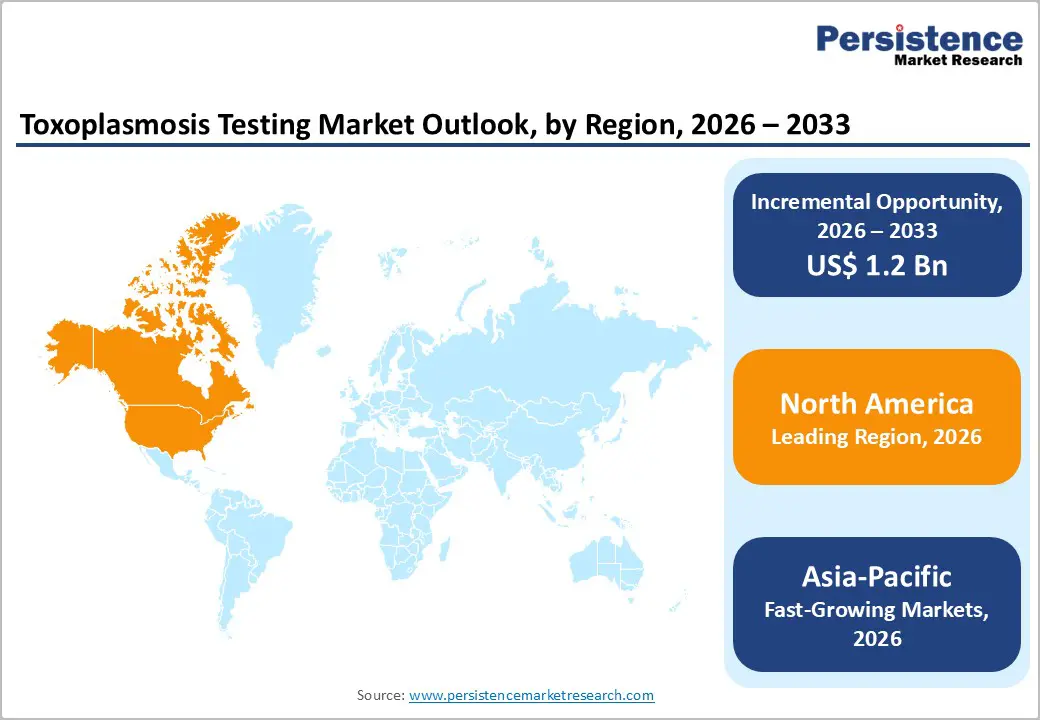
The global toxoplasmosis testing market is expanding steadily, driven by telehealth integration, healthcare analytics, and digital care adoption. North America dominates with advanced healthcare infrastructure and robust regulations. Asia-Pacific is the fastest-growing region, supported by healthcare expansion, government digital initiatives, rising patient awareness, and increased investments in testing services, software, and remote monitoring solutions.
| Report Attribute | Details |
|---|---|
|
Global Toxoplasmosis Testing Market Size (2026E) |
US$ 2.4 Bn |
|
Market Value Forecast (2033F) |
US$ 3.5 Bn |
|
Projected Growth (CAGR 2026 to 2033) |
5.8% |
|
Historical Market Growth (CAGR 2020 to 2025) |
5.0% |
Driver: Growing adoption of point-of-care and rapid diagnostic tests for early detection
The adoption of point?of?care (POC) and rapid diagnostic tests is a key driver of the Toxoplasmosis Testing Market because these tools enable earlier diagnosis at the site of patient care, reducing time to treatment and easing clinical workflow pressures. Traditional serological methods for Toxoplasma gondii rely on laboratory infrastructure and expert personnel, often delaying results. By contrast, POC kits have demonstrated high performance; U.S. trials showed near?100?percent sensitivity and above 96?percent specificity for Toxoplasma?IgG detection across multiple rapid test kits, validating their reliability outside traditional labs.
Strong global seroprevalence further justifies the shift toward rapid diagnostics. Systematic reviews estimate that approximately 36?percent of the global population show exposure to T. gondii, underscoring the widespread burden and the need for accessible testing. In resource?limited settings, rapid POC tests have proved highly sensitive (over 96?percent) and specific (nearly 99?percent), even using simple finger?stick blood, making them viable where laboratory access is constrained. Together, these data from reliable research reinforce how rapid and point?of?care diagnostics are expanding market demand by enabling efficient, accurate early detection in diverse clinical and field settings.
Restraints: Dependence on skilled technicians for laboratory-based diagnostic methods
Laboratory?based Toxoplasmosis diagnostics, such as ELISA, IFAT, and PCR assays, require trained laboratory scientists and technologists to conduct testing, interpret results, and ensure quality control. Across clinical laboratories, vacancy rates of 7–11?percent, and up to 25?percent in some regions, reflect ongoing workforce shortages, increasing workload and burnout among existing staff, which can delay test processing and compromise accuracy. In the United States alone, nearly 39?percent of lab professionals cite limited staff as a primary operational challenge, affecting turnaround times and test reliability.
Globally, the shortage of trained laboratory personnel is compounded by insufficient training programs, retirement of experienced professionals, and uneven workforce distribution, particularly in low?resource areas where laboratories often depend on underqualified technicians. In countries like Pakistan, only around 7?000 qualified medical lab technologists serve diverse diagnostic needs, forcing some facilities to rely on less?trained staff, reducing diagnostic precision. This dependency on skilled technicians for accurate Toxoplasmosis testing constrains market growth by limiting testing capacity, increasing operational costs, and creating access disparities in underserved regions.
Opportunity: Development and adoption of rapid, point-of-care, and home-based Toxoplasmosis test kits
The development and uptake of rapid, point?of?care (POC), and home?based Toxoplasmosis test kits represent a significant market opportunity by making diagnostics more accessible outside traditional laboratory settings. Studies of lateral?flow POC tests for Toxoplasma gondii demonstrate high accuracy; for instance, multiple POC kits exhibited 100?percent sensitivity for IgG detection and up to 98.8?percent specificity, comparable to standard lab tests, with results available in 20–30?minutes using simple finger?stick blood. These rapid formats reduce dependence on venous draws, cold chain logistics, and complex equipment, improving access in primary care and community settings.
Given the high global burden of toxoplasmosis, with pooled seroprevalence estimated at approximately 36?% worldwide, particularly among pregnant women at risk of congenital transmission, demand for rapid diagnostics is clear. Early identification through POC or home testing can support timely intervention in prenatal care and immunocompromised populations, aligning with public health goals to reduce morbidity. The simplicity and speed of these tests also support large?scale screening programs and telehealth integration, potentially expanding market reach in low?resource and remote regions.
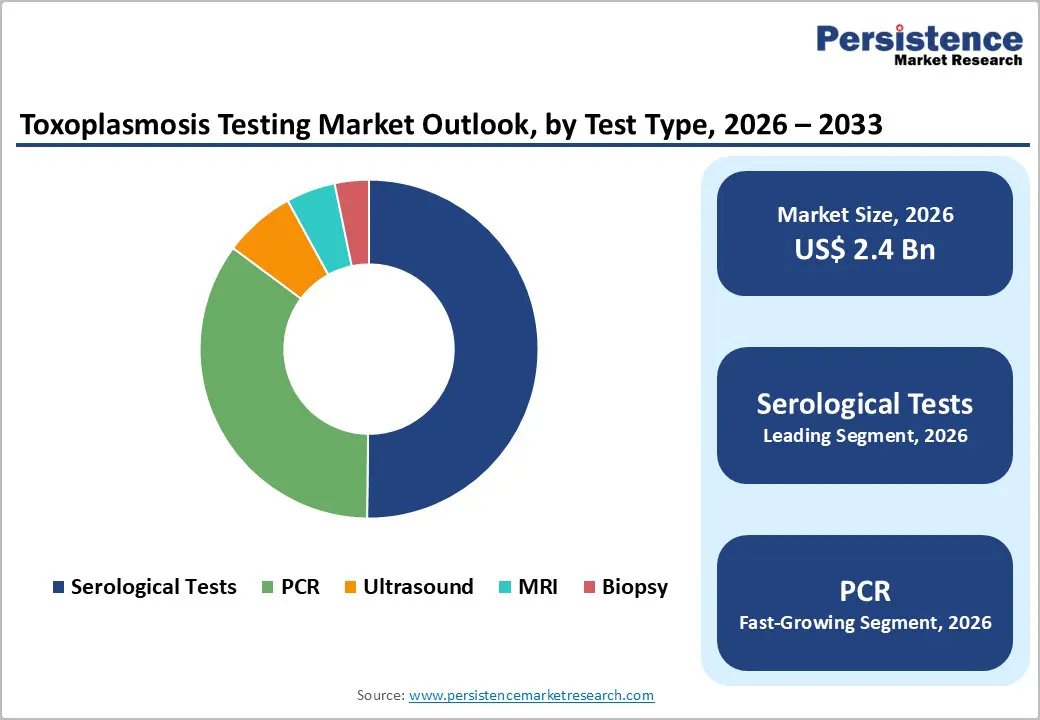
By Test Type, Serological Tests Dominates the Toxoplasmosis Testing Market
Serological Tests occupies 50.2% share of the global market in 2025, because they remain the most accessible, reliable, and broadly applicable diagnostic approach for detecting Toxoplasma gondii infection across clinical settings. These assays, such as ELISA and immunoassays, identify specific IgG and IgM antibodies with high sensitivity (often 89.7–100?% for IgG) and specificity (generally 91.3–100?% for enzyme immunoassays), outperforming many molecular methods for routine screening and large?scale surveillance. Serological testing is recommended by major health authorities as the first?line diagnostic method because antibody detection can indicate current or past infection, is cost?effective, and does not require the sophisticated infrastructure or high technical expertise that PCR and other molecular diagnostics demand.
By Sample, Blood Samples dominates due to accessibility, reliability, and widespread use in antibody detection
Blood samples dominate the toxoplasmosis testing market because they are the primary and most practical specimen for detecting Toxoplasma gondii infection. Serological assays, including ELISA and immunoassays, rely on blood to measure IgG and IgM antibodies, providing reliable evidence of past or recent infection. Blood collection is minimally invasive, widely accepted in clinical practice, and compatible with rapid, point-of-care, and laboratory-based testing, enabling timely results for prenatal screening, immunocompromised patients, and routine surveillance. Studies indicate around 36% global seroprevalence of T. gondii antibodies in blood donors, highlighting the utility of blood for large-scale population monitoring. This accessibility, reliability, and clinical relevance make blood the dominant sample type.
North America Toxoplasmosis Testing Market Trends
North America dominates the toxoplasmosis testing market with 50.2% share in 2025, due to its advanced healthcare infrastructure, robust diagnostic capacity, and proactive screening practices. The region accounts for about 40?% of global market revenue, reflecting extensive use of serological and molecular testing supported by well?equipped laboratories and widespread clinician awareness. The United States, in particular, emphasizes prenatal and immunocompromised patient screening, driving consistent demand for toxoplasmosis diagnostics. Accessible insurance reimbursement and integration of sophisticated testing into routine care further underpin market leadership. Additionally, public health agencies such as the CDC actively promote awareness and early detection, reinforcing testing uptake despite relatively lower seroprevalence compared to other regions.
Europe Toxoplasmosis Testing Market Trends
Europe is an important region in the toxoplasmosis testing market due to its significant public health focus on prenatal and congenital toxoplasmosis surveillance, robust healthcare systems, and active screening programs. The European Centre for Disease Prevention and Control (ECDC) reported 150 confirmed congenital toxoplasmosis cases in the EU/EEA in 2021, with active screening in countries like France accounting for the majority, underscoring ongoing testing demand. Additionally, seroprevalence studies indicate that approximately 31?percent of pregnant women in Europe have Toxoplasma antibodies, demonstrating a substantial population requiring diagnostic monitoring. These factors, combined with well?established laboratory networks, harmonized clinical guidelines, and public health reporting systems, maintain Europe’s strong role in driving testing volumes and adoption of advanced diagnostic methods.
Asia-Pacific Toxoplasmosis Testing Market Trends
Asia Pacific is the fastest?growing region in the toxoplasmosis testing market because healthcare access, diagnostic capacity, and public health investment are expanding rapidly across diverse, high?population countries. Health expenditure as a share of GDP in several Asia?Pacific nations rose between 2015 and 2021, with China, Philippines, and Nepal increasing capital investment in diagnostic equipment and ICT in healthcare, reflecting broader enhancement of diagnostic infrastructure. Moreover, Toxoplasma gondii prevalence varies widely within the region, with Southeast Asian populations showing rates from about 2?percent up to 49?percent in some groups, underscoring substantial unmet testing demand for effective detection and management. These factors, combined with increased emphasis on early disease diagnosis and rural health access, are driving robust market growth.
Leading toxoplasmosis testing market companies focus on connected devices, software, and services, prioritizing interoperability, security, and ease of use. Investments in AI-driven analytics, telehealth, and patient engagement enhance diagnostics, while R&D improves test accuracy. Collaborations with healthcare providers and regulators accelerate adoption, supporting remote monitoring, digital health integration, and efficient, continuous patient care worldwide.
Key Industry Developments:
The global toxoplasmosis testing market is projected to be valued at US$ 2.4 Bn in 2026.
Rising Toxoplasmosis prevalence, telehealth adoption, rapid diagnostics, AI integration, and expanding healthcare infrastructure drive growth.
The global toxoplasmosis testing market is poised to witness a CAGR of 5.8% between 2026 and 2033.
Rapid, point-of-care, home-based tests, AI analytics, telehealth integration, prenatal screening, and emerging market expansion.
Abbott Laboratories, Bio-Rad Laboratories Inc, Affymetrix Inc., Beckman Coulter Inc., Biotest, Cepheid Inc.
| Report Attribute | Details |
|---|---|
|
Historical Data/Actuals |
2020 – 2025 |
|
Forecast Period |
2026 – 2033 |
|
Market Analysis |
Value: US$ Bn |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
By Test Type
By Sample
By End User
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author