ID: PMRREP35462| 195 Pages | 14 Oct 2025 | Format: PDF, Excel, PPT* | Healthcare

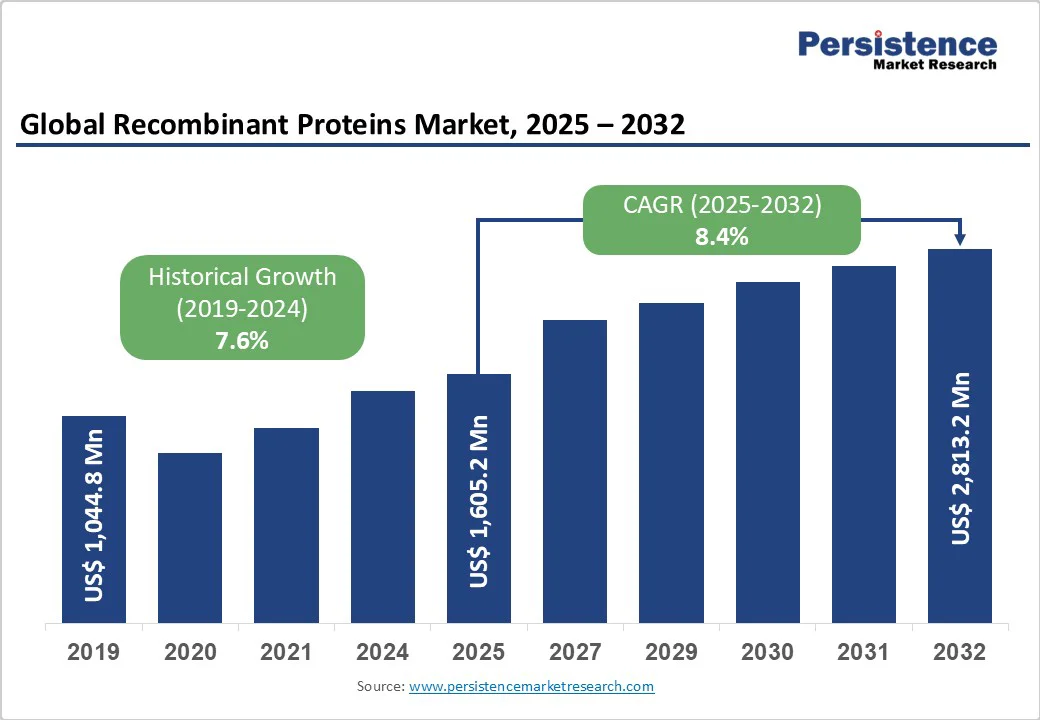
The global recombinant proteins market size is expected to be valued at US$1,605.2 million in 2025 and is projected to witness a CAGR of 8.4% from 2025 to 2032. The market is expected to reach a value of US$2,813.2 million in 2032.
Initially developed for use in insulin and growth hormone therapies, their applications have rapidly expanded to include monoclonal antibodies, cancer immunotherapies, and vaccine development.
Products such as recombinant erythropoietin and interferons have significantly improved treatment outcomes in oncology and hematology.
| Key Insights | Details |
|---|---|
| Recombinant Proteins Market Size (2025E) | US$ 1,605.2 Mn |
| Market Value Forecast (2033F) | US$ 2,813.2 Mn |
| Projected Growth (CAGR 2025 to 2033) | 8.4% |
| Historical Market Growth (CAGR 2019 to 2024) | 7.6% |
The global healthcare sector is experiencing a sharp rise in chronic illnesses like cancer, diabetes, and autoimmune diseases, driving the need for advanced therapeutic options such as recombinant protein-based medications.
The World Health Organization (WHO) reports that chronic diseases are responsible for approximately 74% of worldwide deaths, with cancer and diabetes showing particularly rapid increases. These conditions often demand long-term, precise treatment strategies, positioning recombinant proteins as vital components in contemporary medical care.
Therapies involving recombinant proteins, such as insulin analogs for diabetes and monoclonal antibodies for cancer, provide targeted treatment with fewer adverse effects compared to traditional methods.
For example, monoclonal antibodies such as trastuzumab have transformed the management of HER2-positive breast cancer by selectively attacking cancer cells while minimizing damage to healthy tissues. Likewise, recombinant cytokines, including interferons and interleukins, play a crucial role in controlling autoimmune diseases such as rheumatoid arthritis and multiple sclerosis by regulating the immune system.
In addition to therapeutic uses, recombinant proteins are essential in diagnostics and disease monitoring. Protein-based biomarkers facilitate early detection and enable personalized treatment plans, thereby enhancing patient prognosis. Furthermore, advancements in biomanufacturing techniques have enabled the large-scale production of these complex molecules, thereby lowering costs and improving their availability in various healthcare settings.
A major technical challenge in the recombinant proteins market is ensuring correct protein folding and preserving structural integrity during and after production. Misfolded proteins often lose their biological activity and can aggregate, reducing efficacy and potentially causing immune responses when used therapeutically.
According to the National Institutes of Health (NIH), approximately 30% of recombinant proteins expressed in Escherichia coli form inclusion bodies due to improper folding, necessitating complex and costly refolding procedures to regain functionality.
The U.S. Food and Drug Administration (FDA) emphasizes that maintaining protein structural stability is critical for therapeutic safety and effectiveness, as even minor conformational changes can disrupt receptor binding or trigger adverse immune reactions.
These issues are especially challenging for complex proteins such as antibodies, enzymes, and growth factors that require precise post-translational modifications, such as disulfide bond formation or glycosylation processes, not supported by all expression systems.
As a result, manufacturers must invest significant effort in optimizing expression methods, developing efficient refolding techniques, and performing comprehensive stability testing. These demands lead to extended production timelines and higher costs, which in turn limit the commercial scalability of many recombinant protein products, particularly in settings with limited resources or urgent needs.
The advancement of custom-designed recombinant proteins is revolutionizing precision medicine, especially for rare diseases where traditional treatments often prove inadequate. This approach involves creating highly specific proteins engineered to perform targeted functions, effectively addressing the distinct biological mechanisms of each condition.
A major benefit of these custom recombinant proteins is their capacity to correct particular genetic or enzymatic defects. For instance, in rare metabolic disorders such as lysosomal storage diseases, engineered enzymes can replace defective proteins, restoring normal cellular activity and easing symptoms. Unlike generalized drugs, these proteins target the underlying cause directly, reducing unintended effects and lowering the risk of adverse reactions.
Furthermore, advancements in computational biology and the development of gene-editing tools, such as CRISPR, have enabled the design of protein variants with enhanced stability, solubility, and binding affinity, thereby boosting their therapeutic potential. For example, engineered recombinant proteins aimed at faulty gene pathways have demonstrated encouraging outcomes in preclinical studies for certain genetic muscle disorders.
In oncology, another promising field, custom proteins are being developed to strengthen the immune system’s ability to detect and eliminate cancer cells. Through the engineering of checkpoint inhibitors and bispecific antibodies, therapies can be precisely tailored to a patient’s specific cancer profile, resulting in improved treatment outcomes.
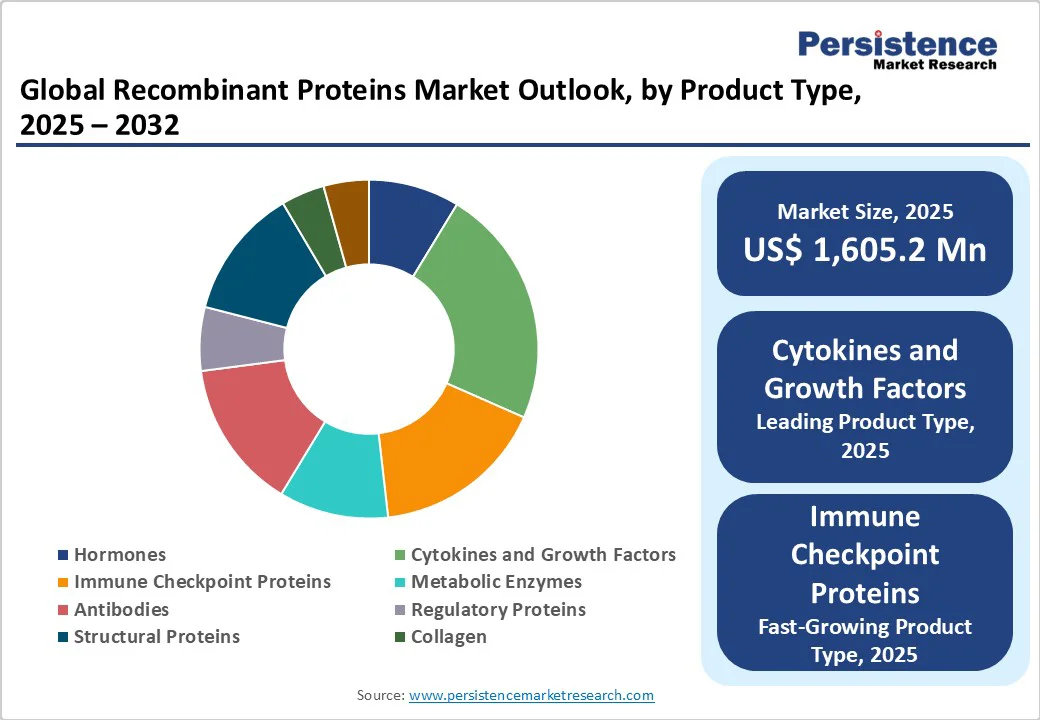
Cytokines and growth factors lead the recombinant proteins market due to their vital roles in immune regulation, cell growth, and tissue repair, making them indispensable in the treatment of cancer, autoimmune, and inflammatory diseases.
The U.S. National Institutes of Health (NIH) highlights over 1,000 clinical trials focusing on cytokine-based therapies for cancer and infections. Growth factors like erythropoietin and granulocyte colony-stimulating factor (G-CSF) are extensively used to treat chemotherapy-induced anemia and neutropenia, boosting demand.
The Centers for Disease Control and Prevention (CDC) reports that cancer and autoimmune diseases are major global health burdens, increasing the need for cytokine and growth factor therapies. While hormones and antibodies have important roles, the wide therapeutic applications and functional diversity of cytokines and growth factors give them a dominant market position in recombinant proteins.
Mammalian expression systems dominate the recombinant proteins market primarily due to their ability to produce complex proteins with proper folding, post-translational modifications (PTMs), and biological activity essential for therapeutic use.
According to the U.S. Food and Drug Administration (FDA), many biologics, including monoclonal antibodies and hormones, require glycosylation and disulfide bonding, which can only be achieved in mammalian cells, such as CHO (Chinese Hamster Ovary) cells. This ensures higher efficacy and safety of protein drugs. In contrast, bacterial systems such as Escherichia coli lack the machinery for these modifications, limiting their use to simpler proteins.
The National Center for Biotechnology Information (NCBI) notes that mammalian systems account for over 70% of commercial therapeutic protein production, primarily due to their superior product quality. Yeast and insect cell systems offer intermediate complexity but cannot fully replicate human-like PTMs, keeping mammalian systems as the preferred choice in pharmaceuticals.
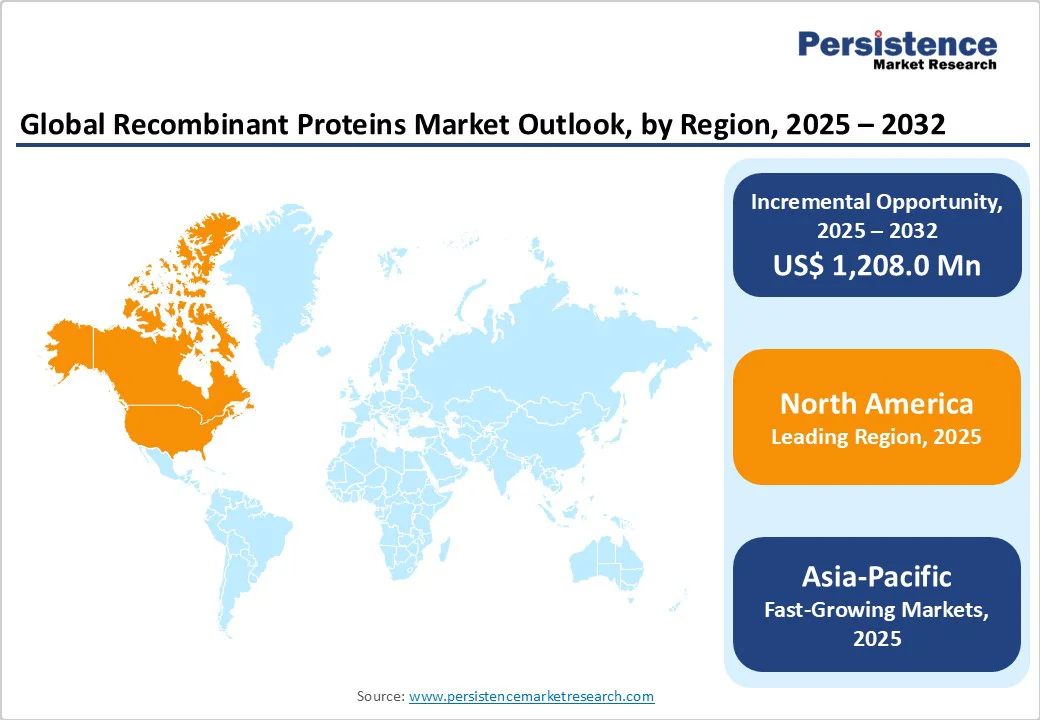
North America, particularly the U.S., leads the recombinant proteins market due to its robust biomedical research ecosystem, significant public funding, and strong regulatory support. The National Institutes of Health (NIH) allocates over $45 billion annually, with a significant portion supporting biotechnology and protein-based therapeutic research.
The U.S. Food and Drug Administration (FDA) consistently approves a high number of recombinant biologics, including monoclonal antibodies and hormone therapies. Additionally, the Biologics Price Competition and Innovation Act (BPCIA) has facilitated biosimilar approvals, enhancing access. With advanced infrastructure, leading academic institutions, and numerous clinical trials involving recombinant proteins, North America continues to drive innovation and production in this field.
Europe plays a pivotal role in the recombinant proteins market due to its strong biomedical research base, advanced healthcare systems, and active regulatory landscape. The European Union funds biotechnology through programs like Horizon Europe, which allocated €95.5 billion for research between 2021 and 2027, with a major focus on health and biotechnology.
The European Medicines Agency (EMA) has approved numerous recombinant biologics, including monoclonal antibodies and therapeutic enzymes, facilitating market growth. Countries like Germany, France, and the Netherlands are home to leading biotech firms and research institutions. Additionally, Europe accounts for a significant share of global clinical trials involving recombinant proteins, reflecting its commitment to therapeutic innovation.
The Asia Pacific is emerging as a key region in the recombinant proteins market, driven by the rapid expansion of clinical trials, strong government R&D funding, and growing biotech capabilities.
Between 2008 and 2017, the number of registered clinical trials in Asia increased sevenfold, outpacing those in North America and Europe. Public investment also surged: China launched a Green Biological Manufacturing R&D initiative (US$3.1 million), while Singapore is committed to spending US$144 million on protein development programs.
Additionally, leading institutions such as RIKEN (Japan) maintain extensive biobanks that support protein research. These factors accelerated trial activity, substantial public funding, and research infrastructure positioning the Asia Pacific as a dynamic hub for recombinant protein innovation and production.
The global recombinant proteins market is intensely competitive, led by major companies such as Thermo Fisher Scientific, Inc., Merck KGaA, STEMCELL Technologies, GenScrip. These industry leaders prioritize innovation, form strategic collaborations, and broaden their product portfolios to maintain market leadership.
Ongoing clinical trials and regulatory clearances play a crucial role in shaping their market presence. Additionally, initiatives aimed at improving drug accessibility and cost-effectiveness further strengthen their competitive edge in the evolving market landscape.
The global recombinant proteins market is estimated to increase from US$ 1,605.2 Mn in 2025 to US$ 2,813.2 Mn in 2032.
Rising chronic diseases, biopharmaceutical demand, technological advances, expanding therapeutic applications, and growing diagnostics drive market growth globally.
The recombinant proteins market is projected to record a CAGR of 8.4% during the forecast period from 2025 to 2032.
Opportunities include personalized medicine, novel expression technologies, expanding diagnostics, emerging markets, and growing applications in agriculture and industrial biotechnology.
Major players include Thermo Fisher Scientific, Inc., Merck KGaA, STEMCELL Technologies, GenScript, Abcam Limited, Bio-Techne, Miltenyi Biotec and Others.
| Report Attribute | Details |
|---|---|
| Historical Data/Actuals | 2019 - 2024 |
| Forecast Period | 2025 - 2032 |
| Market Analysis | Value: US$ Mn, Volume: As applicable |
| Geographical Coverage |
|
| Segmental Coverage |
|
| Competitive Analysis |
|
| Report Highlights |
|
By Product Type
By Recombinant Protein Expression Systems
By Application
By End-user
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author