ID: PMRREP31310| 199 Pages | 5 Dec 2025 | Format: PDF, Excel, PPT* | Healthcare

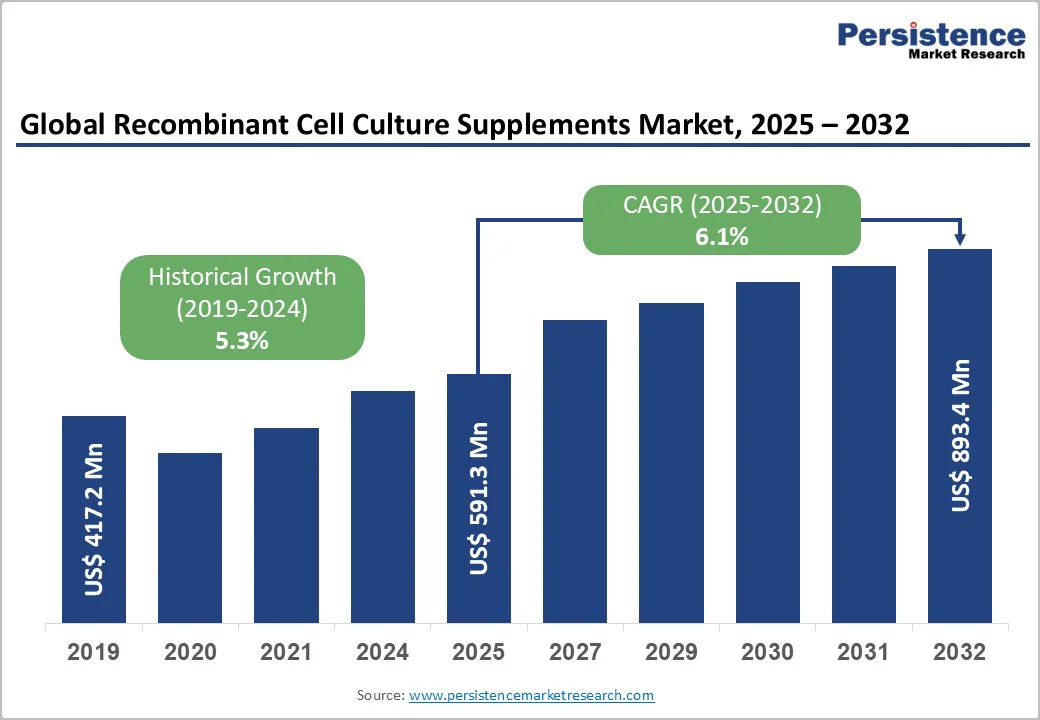
The global recombinant cell culture supplements market size is valued at US$591.3 million in 2025 and projected to reach US$893.4 million by 2032. The market is projected to record a CAGR of 6.1% during the forecast period from 2025 to 2032. The biopharmaceutical companies are shifting toward safer, animal-free, and highly consistent ingredients for cell growth and protein production.
Rising biologics development, increasing monoclonal antibody manufacturing, and the need for scalable upstream processes are driving adoption of cell culture practices. Recombinant supplements offer improved batch-to-batch consistency, reduced contamination risks, and enhanced cell viability, making them essential for modern GMP workflows. Growing demand for viral vectors, vaccines, and advanced therapies such as CAR-T and gene therapies further accelerates market growth.
| Key Insights | Details |
|---|---|
|
Recombinant Cell Culture Supplements Market Size (2025E) |
US$591.3 Mn |
|
Market Value Forecast (2032F) |
US$893.4 Mn |
|
Projected Growth (CAGR 2025 to 2032) |
8.1% |
|
Historical Market Growth (CAGR 2019 to 2024) |
7.3% |
Conventional serum-based media, particularly those derived from fetal bovine serum (FBS), carry risks of contamination with viruses, mycoplasma, and prions. These risks are especially critical in the production of biologics where contamination can lead to batch failures and regulatory complications. Serum-free media, supplemented with recombinant growth factors, cytokines, and hormones, provide a consistent and contamination-free alternative. Adoption rates of serum-free media increased by 25% between 2019 and 2023, owing to rising demand for ethical and contamination-free solutions.
Use of FBS and other animal-derived supplements faces increased scrutiny owing to ethical concerns regarding animal welfare. Regulatory agencies like the FDA and EMA have been advocating for the use of animal-free products in biopharmaceutical manufacturing to ensure ethical and consistent production practices. For instance,
The European Union’s Directive 2010/63/EU on animal welfare has prompted manufacturers to adopt recombinant alternatives. Serum-free media ensures consistent composition, eliminating batch-to-batch variability associated with serum-based media. Recombinant supplements enhance the scalability of cell culture processes by providing reliable performance across different production scales. Serum-free formulations can be tailored for specific cell types, enhancing growth rates and productivity.
Cell culture systems used to produce recombinant proteins, growth factors, and other supplements are highly sensitive to environmental conditions, and contamination can have serious consequences. Risk of contamination is multifaceted and can arise from various sources, including microorganisms, chemical impurities, and human error.
One of the most common types of contamination in recombinant cell culture systems is microbial contamination. Even small amounts of microbial contamination can lead to significant product loss and affect the integrity of cell culture systems. Mycoplasma contamination alone is responsible for up to 50% of contamination incidents in cell culture-based processes, which can lead to incorrect results in research or loss of product batches in manufacturing.
Tissue engineering addresses the critical shortage of donor organs and the growing demand for regenerative treatments. Regenerative medicine is heavily reliant on advanced cell culture techniques, including recombinant supplements, to develop engineered tissues and organs. According to the Global Observatory on Donation and Transplantation, there were over 150,000 organ transplants globally in 2022, but this accounted for less than 10% of the demand, highlighting a significant gap.
Tissue engineering requires precise and contamination-free environments for cell growth and tissue formation. This makes recombinant growth factors, cytokines, and proteins indispensable. Recombinant supplements ensure consistent quality while decreasing variability compared to traditional serum-based media, which is critical for generating functional tissue constructs.
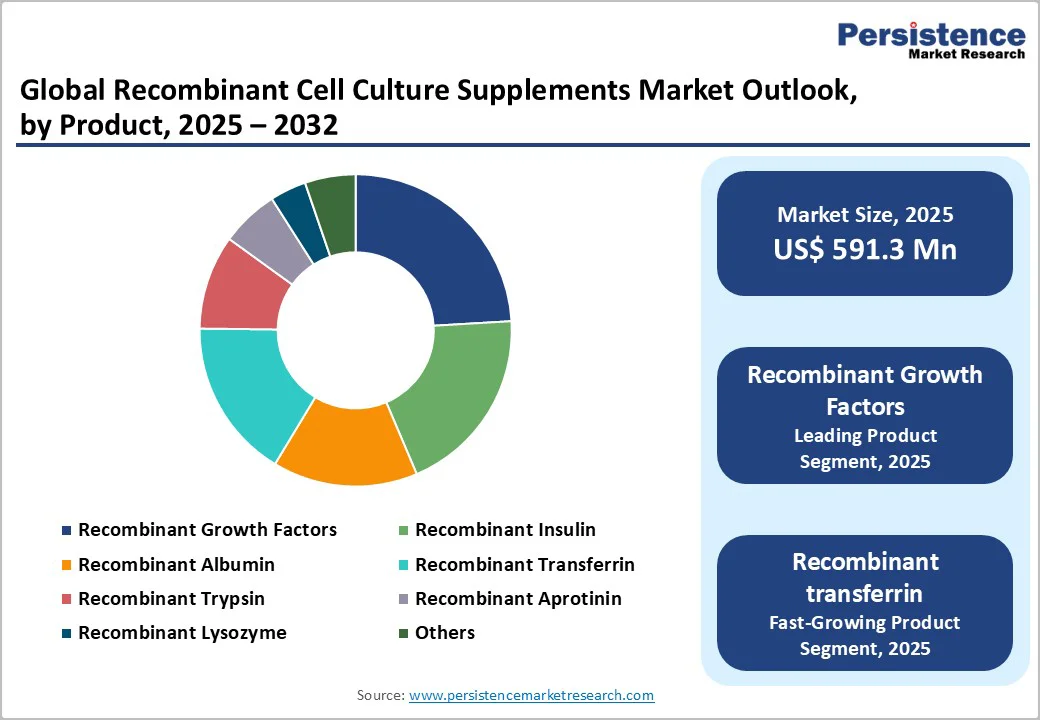
Recombinant growth factors hold the highest market share because they are essential drivers of cell proliferation, differentiation, and productivity across nearly all biopharmaceutical manufacturing platforms. Their critical role in maintaining robust and scalable upstream processes makes them indispensable in serum-free and chemically defined media. As biologics, vaccines, stem-cell therapies, and gene therapies expand, demand for precise, high-purity growth factors continues to rise. These supplements significantly improve cell performance, reduce variability, and support regulatory compliance by eliminating animal-derived components. Their wide applicability across CHO, HEK293, stem cells, and primary cells further strengthens their dominance as core components of advanced cell culture systems.
Biopharmaceutical companies account for the highest share because they are the primary users of recombinant cell culture supplements for large-scale biologics manufacturing. These companies rely on high-purity, animal-free recombinant ingredients to support consistent, GMP-compliant production of monoclonal antibodies, vaccines, cell therapies, and gene therapies. Their extensive investment in upstream process optimization, high-volume cell culture operations, and regulatory-driven demand for safer, traceable components further strengthens their dominance. With expanding pipelines in oncology, immunology, and rare diseases, biopharma companies continuously adopt advanced supplements to enhance yield, reduce variability, and improve process efficiency, making them the largest end-use segment in the market.
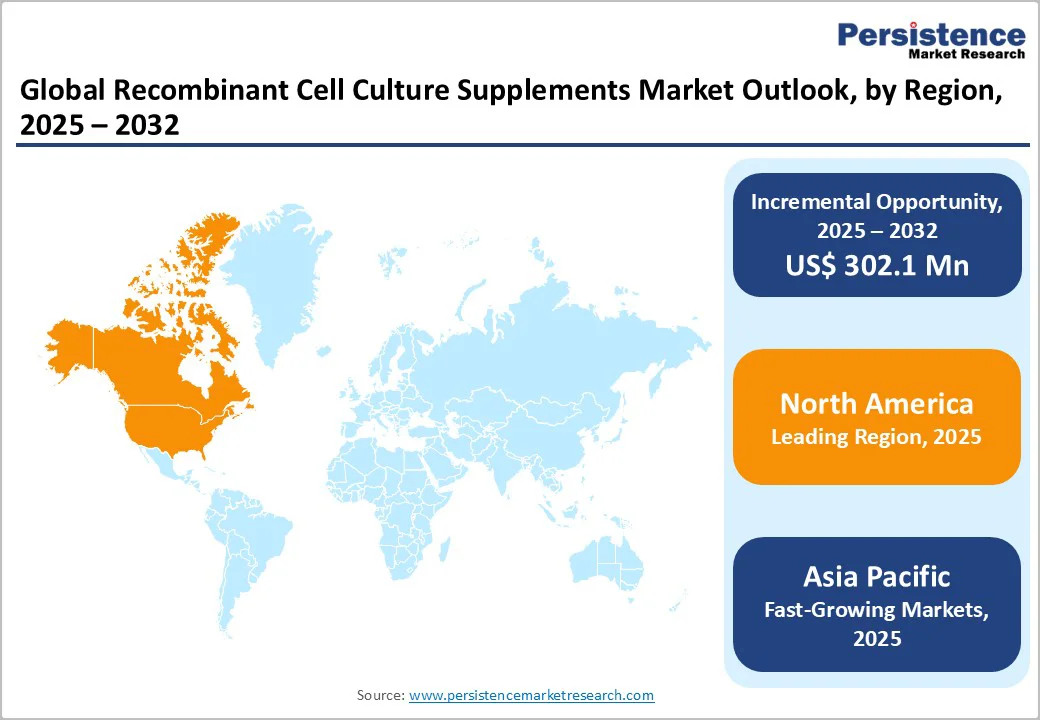
North America leads the recombinant cell culture supplements market due to its strong biopharmaceutical manufacturing ecosystem, advanced R&D infrastructure, and rapid adoption of serum-free and animal-component–free media. The region benefits from substantial investments in biologics, monoclonal antibodies, and cell- and gene-therapy pipelines, driving consistent demand for high-purity recombinant supplements. The U.S. dominates regional growth, supported by a large network of biotech companies, CDMOs, and academic research centers. Strong FDA regulatory frameworks encourage the use of safer, well-defined recombinant components, while continuous innovation in stem cell research, vaccine development, and viral vector production further reinforces North America’s leadership.
The Asia Pacific recombinant cell culture supplements market is emerging rapidly due to expanding biologics manufacturing, growing investments in biopharma infrastructure, and increasing adoption of serum-free production systems. Countries such as China, India, South Korea, and Singapore are strengthening their biotech ecosystems through government funding, research incentives, and the establishment of new GMP-compliant facilities. Rising demand for vaccines, biosimilars, and cell- and gene-therapy research is accelerating the need for high-quality recombinant supplements. Local CDMOs and academic institutes are increasingly transitioning to animal-free, consistent formulations, making Asia Pacific one of the fastest-growing regions in advanced cell culture technologies.
Companies in the recombinant cell culture supplements market are innovating their product offerings to stay relevant. They are progressively investing in research and development activities to develop new and improved recombinant cell culture supplements with enhanced performance, purity, and specificity.
Businesses are focusing on expanding their product portfolios to cater to a wide range of applications. They are introducing specialized supplements to support the culture of stem cells and other cell types used in these therapies.
Manufacturers are launching new supplements that facilitate tissue regeneration and scaffold-based growth. They are focused on providing cell culture supplements that support the growth of mammalian cells used in large-scale biologics production.
The global market is projected to be valued at US$591.3 Mn in 2025.
Key drivers of the Global Recombinant Cell Culture Supplements Market include the shift toward serum-free and chemically defined media, growing biologics and biosimilars production, and rising demand for high-quality, animal-component-free–free ingredients.
The global market is poised to witness a CAGR of 6.1% between 2025 and 2032.
Key opportunities in the Global Recombinant Cell Culture Supplements Market include expanding demand for cell- and gene-therapy manufacturing, increasing adoption of serum-free and animal-free formulations, and rising investments in biologics and biosimilar production.
Merck KGaA, Thermo Fisher Scientific Inc., GE Healthcare, Lonza Group AG, F. Hoffmann-La Roche, and others
| Report Attribute | Details |
|---|---|
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis |
Value: US$ Mn |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
By Product
By Source
By Application
By End User
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author