ID: PMRREP35531| 189 Pages | 30 Jul 2025 | Format: PDF, Excel, PPT* | Healthcare

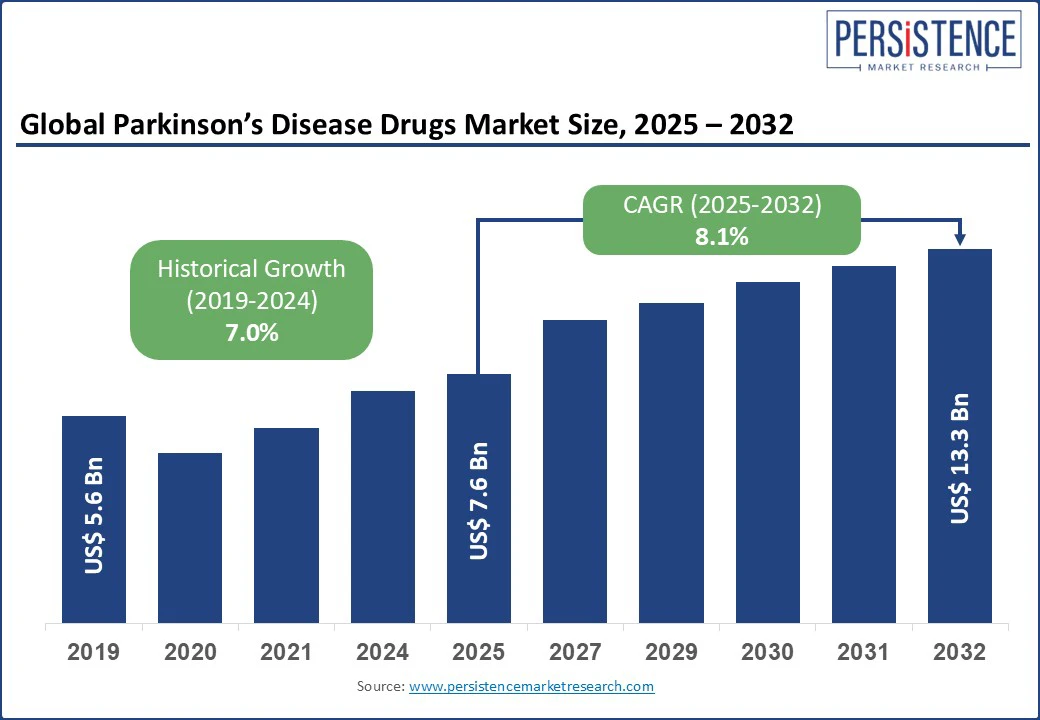
The global parkinson’s disease drugs market size is likely to be valued at US$ 7.6 Bn in 2025 and is estimated to reach US$ 13.3 Bn in 2032, growing at a CAGR of 8.1% during the forecast period 2025 - 2032. Rapid transformation, propelled by demographic shifts, therapeutic innovation, and evolving payer dynamics boosts the need for clinical diagnosis for Parkinson disease drugs. As the global patient base expands, stakeholders are witnessing a transition from legacy symptomatic treatments to a diversified portfolio that includes adjunctive therapies, novel delivery systems, and pipeline candidates targeting disease modification.
This evolution is being shaped not only by clinical demand but also by commercial imperatives, including improved reimbursement access. Investors and pharmaceutical companies are positioning Parkinson’s therapeutics as a long-horizon growth opportunity in the broad neurology portfolio.
Key Industry Highlights
|
Global Market Attribute |
Key Insights |
|
Parkinson’s Disease Drugs Market Size (2025E) |
US$ 7.6 Bn |
|
Market Value Forecast (2032F) |
US$ 13.3 Bn |
|
Projected Growth (CAGR 2025 to 2032) |
8.1% |
|
Historical Market Growth (CAGR 2019 to 2024) |
7.0% |
Increasing awareness and early diagnosis initiatives are pushing the Parkinson’s disease drugs market growth by expanding the treatable patient pool and shifting treatment initiation to early stages of the disease. Public health campaigns such as those led by organizations, including the Michael J. Fox Foundation and the Parkinson’s Foundation, have significantly improved recognition of early, non-motor symptoms. Hence, diagnoses are occurring sooner, often when patients are still in their 50s or early 60s, requiring decades of sustained medication management.
The impact of this shift is evident in prescribing trends. A 2022 study published in Parkinsonism & Related Disorders found that patients diagnosed earlier were more likely to start with dopamine-sparing agents. This often delays the requirement for levodopa and potentially reduces long-term motor complications. It has created rising demand for early-stage medications such as rasagiline and pramipexole, primarily in high-awareness markets. Additionally, national screening programs are being piloted in countries with aging populations, thereby bolstering demand.
Adverse events associated with current Parkinson’s disease therapeutics are significantly hampering the field by limiting long-term adherence, narrowing patient eligibility, and pushing hesitancy among clinicians to intensify treatment. One of the most significant challenges lies in levodopa-induced dyskinesia, a common side effect experienced by up to 80% of patients after 5 to 10 years of levodopa therapy. These involuntary movements severely affect quality of life and often compel clinicians to reduce dosages or introduce complex adjunctive regimens.
Dopamine agonists, while often prescribed in early-stage Parkinson’s to delay levodopa initiation, present another layer of risk. Drugs such as pramipexole and ropinirole are strongly associated with Impulse Control Disorders (ICDs), including compulsive gambling, hypersexuality, and binge eating. A recent study published in JAMA Neurology found that up to 17% of Parkinson’s patients on dopamine agonists exhibited at least one form of ICD. These neuropsychiatric side effects have led to surging scrutiny and restricted use in patients with a history of mood disorders or addiction, thereby reducing their therapeutic flexibility.
Expanding reimbursement and insurance coverage is creating new opportunities in the market by improving access to premium drugs and therapies that were previously out of reach for large patient segments. In the U.S., for example, the inclusion of new drugs such as Inbrija and extended-release formulations, including Rytary, under Medicare Part D has significantly improved their uptake. It is evident among elderly patients who form the bulk of the Parkinson’s population.
Private insurance providers in Europe are also beginning to cover high-cost interventions, including apomorphine infusion therapy and Duodopa intestinal gel. These were once limited to only the most affluent or clinically severe cases. In the U.K. and Germany, coverage expansion for device-assisted therapies has resulted in early intervention among patients with advanced motor complications. It is further delaying more invasive procedures such as deep brain stimulation.
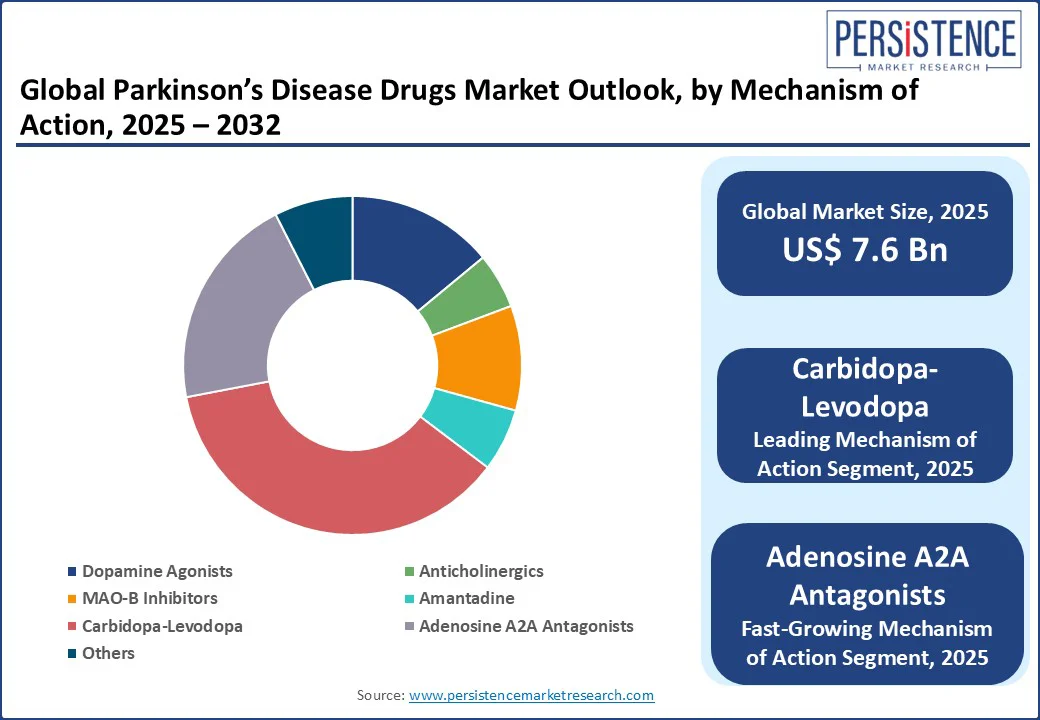
By mechanism of action, the market is divided into dopamine agonists, anticholinergics, MAO-B inhibitors, amantadine, carbidopa-levodopa, and adenosine A2A antagonists. Among these, in 2025, carbidopa-levodopa is expected to hold nearly 36.7% of the Parkinson’s Disease drugs market share as it directly addresses dopamine deficiency, the central pathophysiological hallmark of the disease. Levodopa is a precursor of dopamine that can cross the blood-brain barrier, unlike dopamine itself. However, when administered alone, a large portion of levodopa is converted to dopamine peripherally, leading to side effects such as hypotension. Carbidopa prevents this premature conversion, allowing more levodopa to reach the brain while minimizing systemic side effects.
Adenosine A2A antagonists are gaining traction due to their non-dopaminergic mechanism. This allows them to complement standard dopamine-based therapies without exacerbating dopamine-related side effects. These antagonists target the adenosine A2A receptors, which are densely located in the striatum. By modulating the indirect pathway of the basal ganglia, A2A antagonists help restore the balance of excitatory and inhibitory signals disrupted by dopamine loss. It hence leads to improved motor function.
Based on route of administration, the market is segregated into oral, transdermal, subcutaneous, intranasal, and infusion. Out of these, the oral segment is projected to account for about 74.8% of share in 2025, owing to its compatibility with long-term, chronic treatment and patient-driven dose management. Parkinson’s disease typically requires lifelong medication, and oral formulations allow for convenient self-administration. It is evident in early to mid-stage patients who retain sufficient motor function. This ease of use is significant given the average age of diagnosis is over 60, and adherence becomes increasingly important for disease control over decades.
The transdermal route is seeing a steady growth, backed by its ability to provide continuous drug delivery, which helps smooth out the peaks and troughs associated with oral dosing. It is valuable in advanced stages of the disease, where motor fluctuations and off periods become more pronounced. Transdermal systems also help bypass gastrointestinal absorption, which is often erratic in Parkinson’s patients due to gastroparesis or delayed gastric emptying.
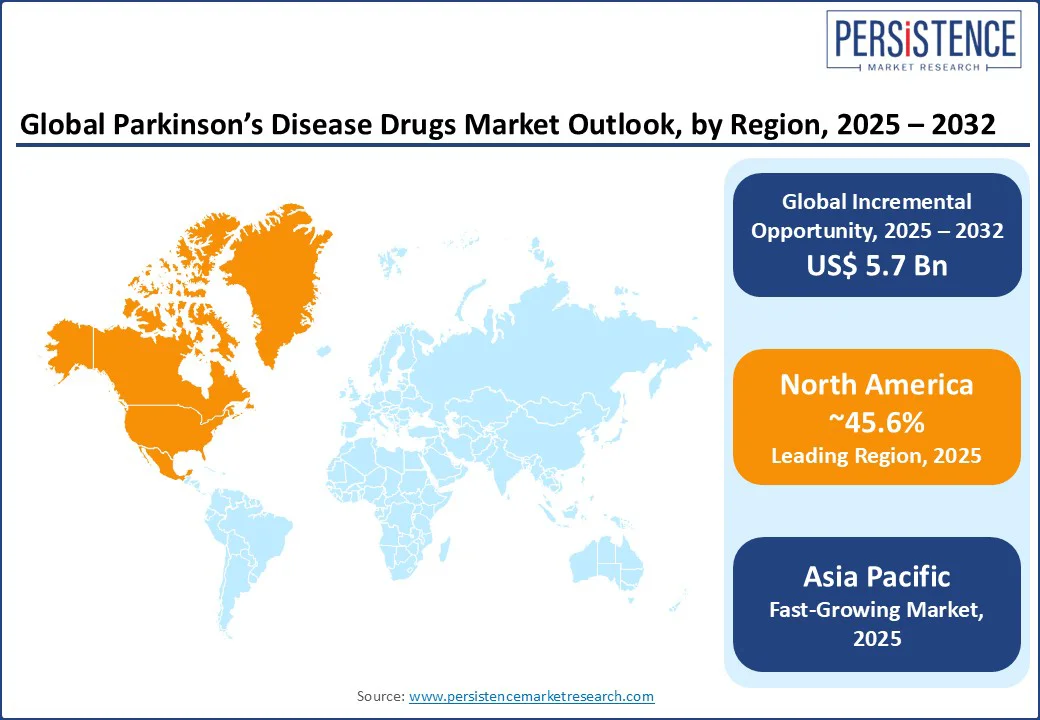
North America is anticipated to account for approximately 45.6% of share in 2025, owing to a high level of therapeutic saturation in symptomatic treatments and a surging push toward disease-modifying approaches. The U.S. Parkinson’s disease drugs market holds the most prominent share, propelled by the high prevalence of the disease. According to Parkinson’s Foundation, an estimated 1.1 million individuals in the U.S. are living with the disease and by 2030, this number is predicted to rise to 1.2 million.
It has resulted in robust demand for both standard dopaminergic therapies and new adjunctive treatments addressing unmet demand such as off episodes and non-motor symptoms. Recent FDA approvals reflect this evolving treatment focus. In the recent past, Acorda Therapeutics, for instance, launched Inbrija, an inhaled levodopa powder. It specifically targets intermittent off episodes, a significant limitation of oral levodopa. Although its uptake was hindered initially due to reimbursement issues, its unique delivery mechanism represents a shift toward patient-centric innovations in the U.S. market.
In Europe, the market is navigating a delicate balance between cost-containment pressures and the rising demand for novel therapies. As per Parkinson’s U.K., nearly 166,000 individuals have been diagnosed with Parkinson’s in the country, and someone is diagnosed every 20 minutes. Hence, there is an increasing urgency for both effective symptom management and neuroprotective solutions. However, Europe’s fragmented healthcare systems and centralized pricing negotiations often delay market access for innovative drugs.
Despite these challenges, several countries in Europe are fostering innovation through public-private partnerships and academic research hubs. For example, the EU-funded Innovative Medicines Initiative (IMI) supports large-scale projects such as IMPRIND. It investigates the propagation of alpha-synuclein pathology. Germany and the U.K. are at the forefront of prescribing new adjunctive treatments. In addition, the European Medicines Agency (EMA) has granted orphan designation and PRIME status to several Parkinson’s pipeline therapies. This highlights the region’s openness to accelerated development paths.
Asia Pacific is witnessing uneven access, rapid epidemiological growth, and a rising emphasis on domestic innovation, mainly in China, Japan, and South Korea. Japan leads the region in terms of innovative therapeutic adoption and drug innovation. Its aging population has made Parkinson’s a public health priority. Domestic players such as Kyowa Kirin have developed notable therapies such as istradefylline (Nourianz), an adenosine A2A receptor. The country also actively supports personalized treatment pathways, including wearable technologies integrated with continuous drug delivery systems.
In China, the market is showcasing a dramatic expansion, spurred by government incentives for local research and development as well as fast-track approvals for neurodegenerative diseases. Players such as Simcere and CSPC Pharmaceutical are now entering the race with generic and innovative formulations. International drugs, including AbbVie’s Duodopa and Acorda’s Inbrija, however, face regulatory and pricing hurdles. South Korea is emerging as a key clinical trial destination due to strong infrastructure and government backing. India, on the other hand, continues to rely on generic dopaminergic drugs, with limited access to latest adjunctive therapies due to affordability constraints.
The global Parkinson’s disease drugs market is characterized by long-established therapies and emerging disease-modifying treatments. Traditional dopaminergic agents continue to lead in terms of prescription volume due to their efficacy in managing motor symptoms. However, their limitations have led to increased research and commercial activity in adjunctive and non-dopaminergic approaches.
The market has also seen increasing diversification with the entry of drugs targeting non-motor symptoms, which are gaining commercial traction. The competitive intensity is rising in the pipeline segment. Biotech companies and pharmaceutical giants are investing in alpha-synuclein-targeted therapies and neuroprotective agents.
The Parkinson’s disease drugs market is projected to reach US$ 7.6 Bn in 2025.
Patient preference for less invasive therapies and increasing government funding for neurological research are the key market drivers.
The Parkinson’s disease drugs market is poised to witness a CAGR of 8.1% from 2025 to 2032.
Integration of digital health tools and favorable regulatory pathways for orphan drugs are the key market opportunities.
AbbVie Inc., GSK plc, and Viatris Inc. are a few key market players.
|
Report Attribute |
Details |
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis |
Value: US$ Bn |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
|
Customization and Pricing |
Available upon request |
By Mechanism of Action
By Route of Administration
By Distribution Channel
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author