ID: PMRREP35400| 192 Pages | 9 Jun 2025 | Format: PDF, Excel, PPT* | Healthcare

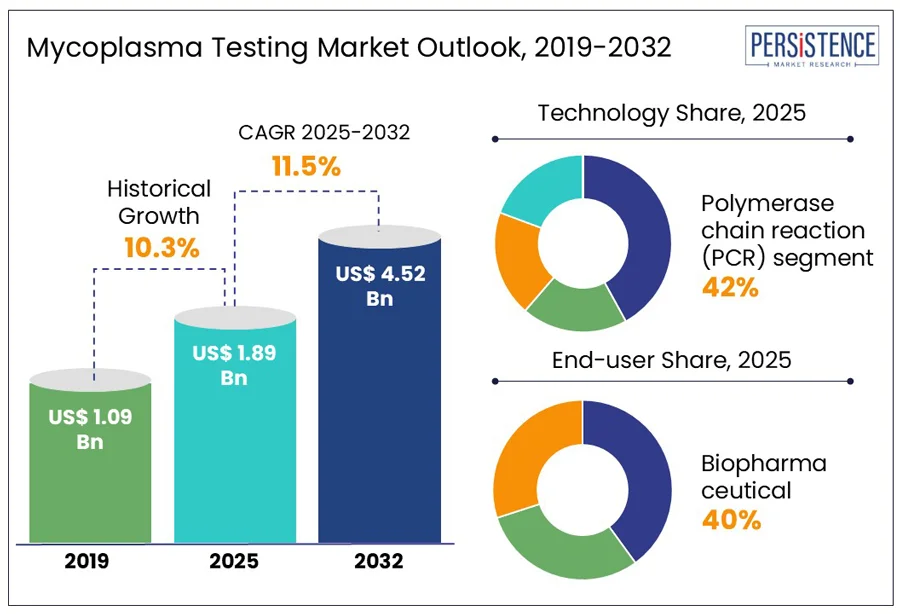
The global mycoplasma testing market size is projected to rise from US$ 1.89 Bn in 2025 to US$ 4.52 Bn by 2032. The market is further anticipated to register a CAGR of 11.5% during the forecast period from 2025 to 2032. According to the Persistence Market Research report, market growth is driven by the prevalence of mycoplasma contamination, expansion of medical and pharmaceutical infrastructure in emerging economies, and supportive government initiatives to enhance bio-manufacturing capacities and biosafety.
Mycoplasma are bacteria (or germs) that can infect different parts of the body. Mycoplasma do not have cell walls and are very small compared to other bacteria, making them resistant to many antibiotics. There are about 200 types of mycoplasma bacteria, but the harmful ones are mycoplasma pneumonia, mycoplasma genitalium, mycoplasma hominis, ureaplasma urealyticum, and ureaplasma parvum. Mycoplasma testing comprises a group of tests that measure antibodies in the blood or detect the microorganism directly through culture or DNA analysis. Mycoplasma testing is also crucial in bio-pharmaceuticals and research laboratories to avoid contamination and wastage of cell cultures. Cell culture contamination is a major concern for biotechnology companies and other research institutes, making mycoplasma testing fundamental.
Key Industry Highlights
|
Global Market Attribute |
Key Insights |
|
Mycoplasma Testing Market Size (2025E) |
US$ 1.89 Bn |
|
Market Value Forecast (2032F) |
US$ 4.52 Bn |
|
Projected Growth (CAGR 2025 to 2032) |
11.5% |
|
Historical Market Growth (CAGR 2019 to 2024) |
10.3% |
The mycoplasma testing market is experiencing significant growth, largely fueled by rising human infection rates and chronic contamination challenges in the biotechnology industry. A study from a leading scientific journal reported a global increase in mycoplasma pneumonia infections, particularly among children, indicating a notable shift in the disease burden after COVID-19. Mycoplasma contamination remains a persistent threat in the biopharmaceutical manufacturing sector. Mycoplasma, due to their small size and lack of a cell wall, often go undetected by traditional microbial monitoring methods. Their presence can compromise the product efficacy and cause safety risks for patients.
Advanced detection solutions, such as the SwiftDx Mycoplasma Detection Kit and the MycoSEQPlus Detection System, have emerged as rapid, sensitive, and compliant testing methods. These technologies enable early detection and intervention, thereby minimizing costly production setbacks and ensuring the safety of biologics, vaccines, and cell-based products. Biopharmaceutical companies are now incorporating testing at multiple points along the production pipeline from raw materials to final product launch.
Real-time PCR-based assays are increasingly favored due to their speed, accuracy, and the ability to detect a wide range of mycoplasma species without cross-reactivity. Leading service providers, such as Eurofins BioPharma Product Testing, are offering assays that adhere with global regulatory standards. With an expanding biopharmaceutical sector, it is crucial to ensure the integrity of cell cultures and biologics through comprehensive mycoplasma testing for protecting both product quality and public health.
The high cost of equipment remains a key restraint in the mycoplasma testing market, particularly in emerging regions including Asia Pacific, Latin America, and Africa, where healthcare budgets and infrastructure are limited, further making advanced diagnostics inaccessible. To alleviate this, innovations such as a miniaturized ELISA device that costs around US$ 1,200 and tests for under US$ 10 are being developed to facilitate inexpensive and point-of-care testing. The Mycoplasma DNA Detection Kit (qPCR) from Eagle Biosciences is priced at US$ 3,365.00, illustrating the high costs often associated with advanced mycoplasma detection technologies; however, it is dedicated only for research use and not for diagnostic applications.
The high cost of these kits, combined with the need for compatible qPCR equipment, creates a significant financial barrier that restricts accessibility. The high equipment and reagent costs impede the widespread adoption of mycoplasma testing, despite its regulatory and quality control importance. The high costs of mycoplasma testing equipment such as PCR systems and microbial culture supplies will also hamper market growth, posing challenges for small and medium size laboratories and research facilities.
Recent advancements in AI-driven mycoplasma testing are revolutionizing quality control in cell therapy and diagnostics. A Springer Nature study discussed a convolutional neural network that detects mycoplasma with greater sensitivity (as few as 5 CFU) and 20-fold faster than traditional methods, significantly reducing manufacturing costs. Similarly, another study by a leading scientific journal presented a GBDT-based AI tool capable of accurately distinguishing pediatric mycoplasma pneumoniae pneumonia using routine blood parameters, improving diagnostic accessibility in resource-limited areas.
Complementing these efforts, Osaka University, partnered with Dai Nippon Printing to develop Japan’s first PMDA-approved AI method for mycoplasma testing in clinical-grade iPS cell-derived cardiomyocytes, aligning with national pharmacopeia standards and minimizing human errors in regenerative medicine. AI minimizes the time and effort needed for manual mycoplasma testing and eradicates the personal bias in image analysis.
A leading scientific journal recommended an AI-supported classification method, combined with portable multispectral fiber-optic reflectometer, to warn and detect eggshell changes caused by mycoplasma synoviae in bird flocks. Egg wholesalers and distributors, veterinary services, sanitary inspection stations, border control agencies could make use of this technology. Together, these advances are transforming the field into a more accurate and accessible specialty.
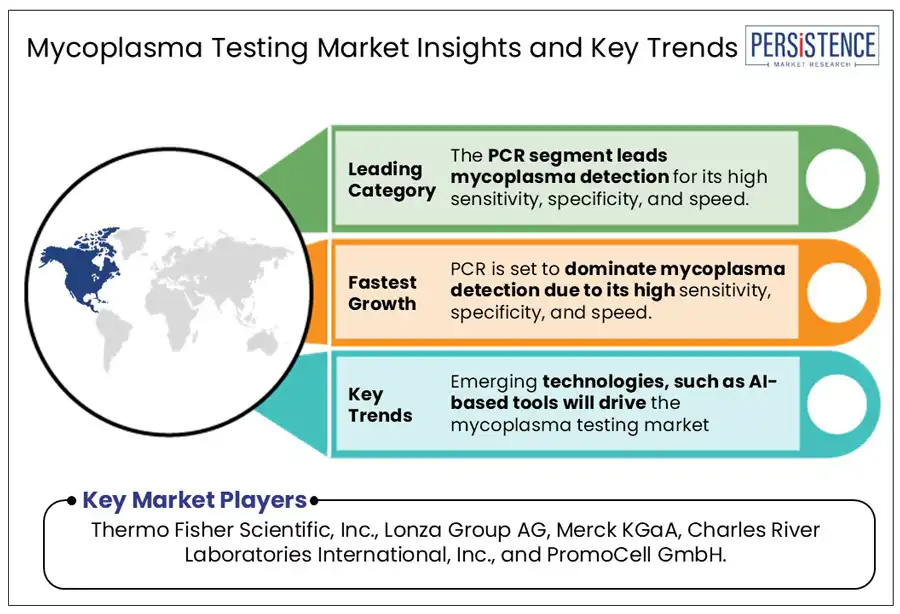
The polymerase chain reaction (PCR) segment is expected to dominate and hold approximately 42% of the market share in 2025. PCR is the most widely used method for mycoplasma detection due to its great sensitivity, specificity, and speed. It works by augmenting specific DNA segments from mycoplasma using thermal cycling and a heat-stable enzyme called Taq polymerase. This technique can detect even minimal contamination and recognize a wide range of mycoplasma species by studying genetic regions such as 16S rRNA. PCR-based kits, such as those from Takara Bio and Merck KGaA, are commonly used in routine cell culture monitoring.
The enzyme-linked immunosorbent assay (ELISA) segment is expected to grow at the fastest rate over the forecast period. ELISA detects mycoplasma antigens using antibody-enzyme interactions. ELISA technique is a simple test with minimal equipment; hence, may lack the sensitivity of PCR and might not detect all mycoplasma species, especially in low-level infections. Depending on lab resources and requirements, combining PCR and ELISA can offer a more comprehensive strategy for ensuring contamination-free cell cultures. For instance, Sigma-Aldrich offers over 1,000 RUO ELISA kits, which include Sandwich, Direct, and Competitive formats to spot soluble protein biomarkers across various species and sample types.
The biopharmaceutical segment is expected to dominate the mycoplasma testing market in 2025, accounting for around 40% of total revenue due to the rising number of research activities among these facilities. The growing incidence of chronic conditions and infectious diseases has surged research activities across pharmaceutical and biotechnology sectors. Companies such as Pfizer and Flagship Pioneering invested US$ 100 Mn in R&D in 2023 to expand drug development efforts, reflecting how pharmaceutical and biotech firms dominate due to strict regulatory requirements and the need to ensure product safety and efficacy. Contamination remains a serious threat during the production of biologics, vaccines, monoclonal antibodies, and gene therapies, making routine testing integral for regulatory compliance with agencies including the FDA, EMA, and ICH. Mycoplasma testing is predominantly crucial during cell line development to guarantee contamination-free production processes.
The contract research organizations (CROs) segment represents the fastest-growing segment in the market. With expanding research services and a strong focus on preclinical testing, CROs rely on mycoplasma testing to maintain sterility and ensure the quality of outsourced services. Their role in supporting biopharmaceutical development continues to grow, fueling further demand for rapid and accurate detection methods. Thermo Fisher Scientific, Charles River Laboratories, Lonza, Merck KGaA, and Eurofins are the renowned CROs.
North America is expected to lead the market with a 46% revenue share, driven by its strong biopharmaceutical and biotechnology sectors. As mycoplasma contamination poses serious risks to the safety and effectiveness of biologics, vaccines, and research outcomes, agencies including the FDA has enforced strict regulations to avoid contamination. The rise in chronic diseases, cancer research, and biologic development has further accelerated the demand for precise and rapid detection methods such as PCR and ELISA. Seasonal respiratory infections, including Mycoplasma pneumoniae, have also increased the need for dependable diagnostics. Organizations including the CDC and NIH, in collaboration with the FDA and HHS, continue to stipulate biological safety testing standards. Key companies such as Charles River Laboratories, Bionique Testing Laboratories, Roche Diagnostics, Lonza Group, and ATCC contribute to market growth.
Canada is anticipated to witness the highest growth over the forecast period, driving increased investments in biomedical research and biomanufacturing. With strict international regulatory standards, there is a high requirement for routine testing of biologics and cell cultures. Canada also partakes in global respiratory disease surveillance, reacting to seasonal trends in infections such as mycoplasma pneumoniae. The adoption of precision medicine and increasing biologic development are highlighting the importance of mycoplasma testing in both academic and industrial research settings.
Asia Pacific mycoplasma testing industry is estimated to grow at the fastest CAGR over the forecast period. Increased implementation of regulations by healthcare organizations to improve biosafety is likely to surge growth opportunities in the future. The National Biotechnology Development Strategy (2020-2025), by the government of India to establish itself as a leading manufacturing entity, is anticipated to positively impact the market. The presence of biological safety guidelines specific to the research segments, such as recombinant DNA research and cell culture, indicates further growth potential. Takeda Pharmaceutical, Eli Lilly, Sanofi, AbbVie, Amgen, AstraZeneca, and Fresenius Kabi are some of the major pharmaceutical companies present in Asia Pacific.
Japan is anticipated to lead in Asia Pacific in 2025, driven by an increasing number of mycoplasma pneumonia cases, with patient numbers reaching the highest levels in the past decade. Increasing incidence of cell culture contamination coupled with the rising demand for rapid and accurate testing is also driving the mycoplasma testing in Japan.
Europe is anticipated to dominate and witness substantial growth in the market from 2025 to 2032. The demand for mycoplasma testing in Europe is growing due to the presence of a robust biopharmaceutical and biotechnology sector. The European Medicines Agency's (EMA) implementation of stringent regulatory standards to ensure safety and efficacy of biological products drive market growth. The increasing investments in biotechnology and life sciences research across Europe have also increased the demand for advanced mycoplasma testing. The EU’s Horizon Europe program, which funds innovative scientific research, focuses on the importance of high-quality standards in biomedical research, driving the adoption of advanced mycoplasma detection technologies, such as qPCR and Next-Generation Sequencing (NGS).
The U.K. is expected to lead the market in terms of market share and revenue and will dominate during the forecast period. Leading companies include Abcam plc and Eurofins Scientific. The presence of a well-established healthcare infrastructure and a robust biopharmaceutical sector encourage a high rate of mycoplasma testing.
The global mycoplasma testing market is highly competitive, with global and domestic players offering a wide range of products and competing for a higher market share. Companies are investing in R&D and adopting growth strategies such as product innovations, strategic partnerships, and acquisitions.
The global market is projected to be valued at US$ 1.89 Bn in 2025.
The industry is driven by the increasing human infection rates and relentless contamination challenges within the biotechnology industry.
The market is poised to witness a CAGR of 11.50% from 2025 to 2032.
AI is increasingly being integrated into mycoplasma testing, offering faster, cheaper, and more accurate interpretations of pathology data.
Major players in the mycoplasma testing industry include Thermo Fisher Scientific, Inc., Lonza Group AG, Merck KGaA, Charles River Laboratories International, Inc., and PromoCell GmbH.
|
Report Attribute |
Details |
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis |
Value: US$ Bn |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
|
Customization and Pricing |
Available upon request |
By Technology
By End-user
By Product & Service
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author