- Executive Summary
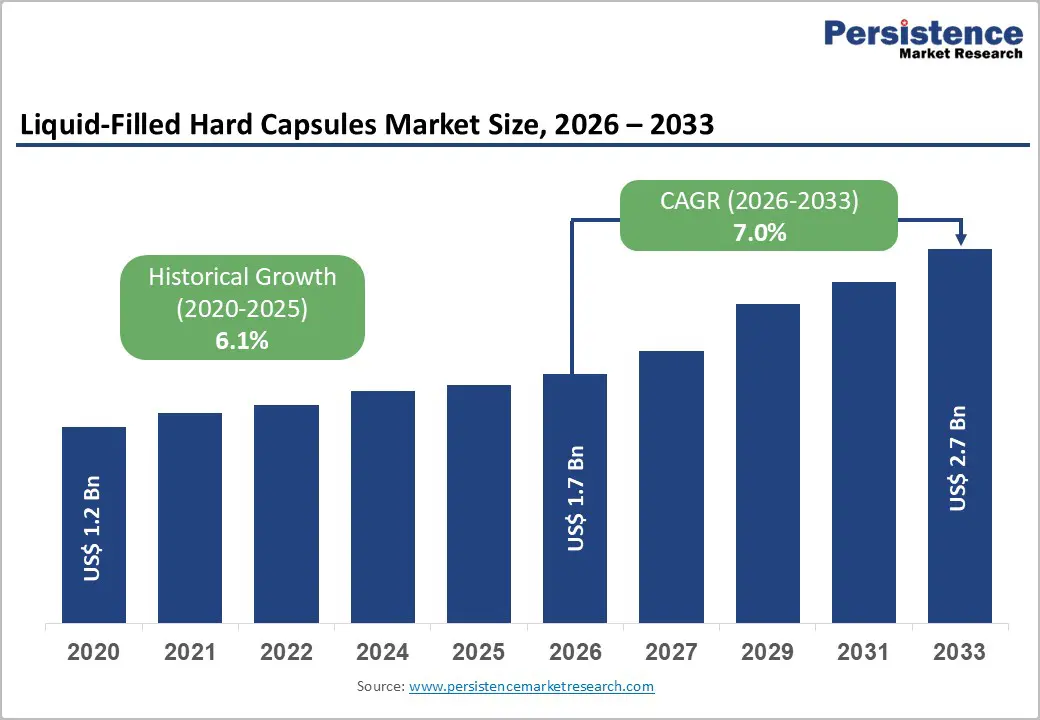
- Global Liquid-Filled Hard Capsules Market Snapshot 2026 and 2033
- Market Opportunity Assessment, 2026-2033, US$ Bn
- Key Market Trends
- Industry Developments and Key Market Events
- Demand Side and Supply-Side Analysis
- PMR Analysis and Recommendations
- Market Overview
- Market Scope and Definitions
- Market Dynamics
- Driver
- Restraint
- Opportunities
- Trends
- Macro-Economic Factors
- Global GDP Outlook
- Global Prison Growth Outlook
- Global Prison Population by Country
- Global Private Prison Market Growth Outlook
- Forecast Factors – Relevance and Impact
- COVID-19 Impact Assessment
- Value Added Insights
- Value Chain analysis
- Key Market Players
- Product Adoption Analysis
- Key Promotional Strategies by key players
- PESTLE Analysis
- Porter's Five Forces Analysis
- Regulatory and Technology Landscape
- Global Liquid-Filled Hard Capsules Market Outlook: Historical (2020 – 2025) and Forecast (2026 – 2033)
- Key Highlights
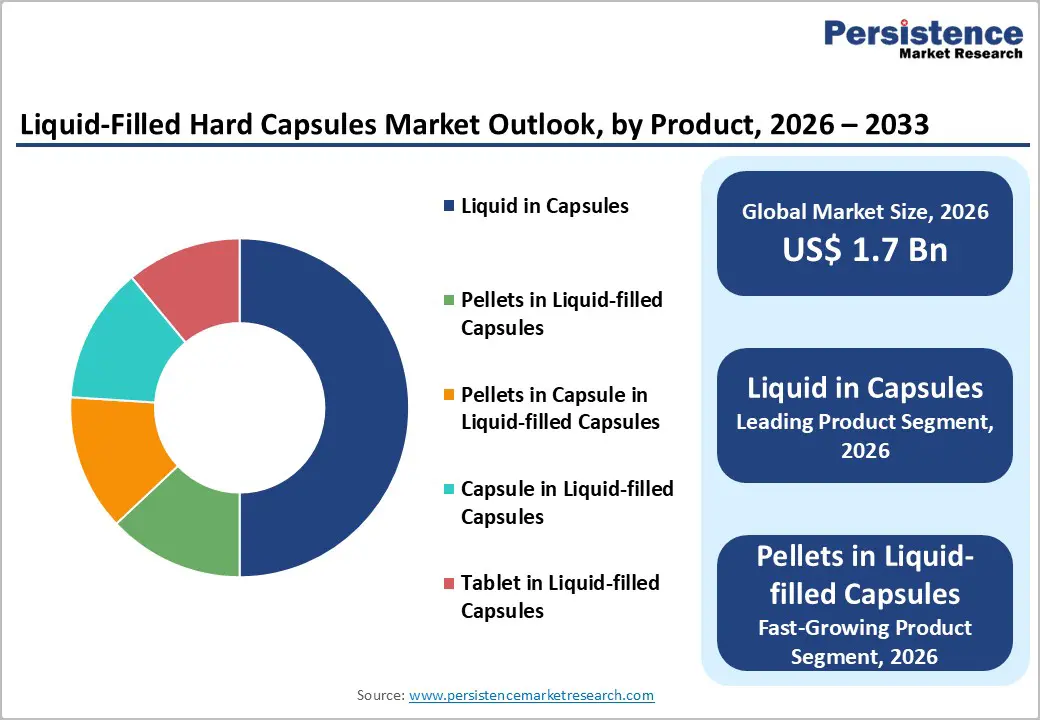
- Global Liquid-Filled Hard Capsules Market Outlook: Product
- Introduction/Key Findings
- Historical Market Size (US$ Bn) Analysis by Product, 2020-2025
- Current Market Size (US$ Bn) Forecast, by Product, 2026-2033
- Liquid in Capsules
- Pellets in Liquid-filled Capsules
- Pellets in Capsule in Liquid-filled Capsules
- Capsule in Liquid-filled Capsules
- Tablet in Liquid-filled Capsules
- Market Attractiveness Analysis: Product
- Global Liquid-Filled Hard Capsules Market Outlook: Raw Material
- Introduction/Key Findings
- Historical Market Size (US$ Bn) Analysis by Raw Material, 2020-2025
- Current Market Size (US$ Bn) Forecast, by Raw Material, 2026-2033
- Gelatin
- Hypromellose Capsules (HPMCs)
- Market Attractiveness Analysis: Raw Material
- Global Liquid-Filled Hard Capsules Market Outlook: Application
- Introduction/Key Findings
- Historical Market Size (US$ Bn) Analysis by Application, 2020-2025
- Current Market Size (US$ Bn) Forecast, by Application, 2026-2033
- Cough & Cold Preparations
- Cardiovascular Therapy Drugs
- Health Supplements
- Vitamin & Dietary Supplements
- Other
- Market Attractiveness Analysis: Application
- Global Liquid-Filled Hard Capsules Market Outlook: End User
- Introduction/Key Findings
- Historical Market Size (US$ Bn) Analysis by End User, 2020-2025
- Current Market Size (US$ Bn) Forecast, by End User, 2026-2033
- Pharmaceutical Companies
- Nutraceutical Companies
- Cosmeceutical Companies
- Contract Manufacturing Organizations
- Market Attractiveness Analysis: End User
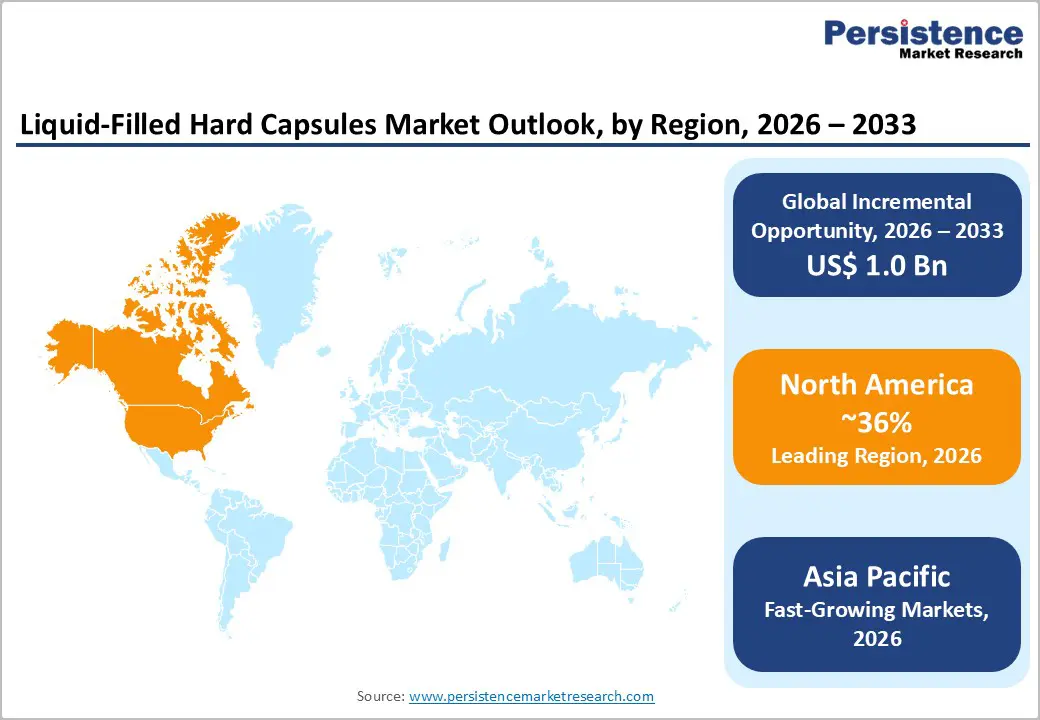
- Global Liquid-Filled Hard Capsules Market Outlook: Region
- Key Highlights
- Historical Market Size (US$ Bn) Analysis by Region, 2020-2025
- Current Market Size (US$ Bn) Forecast by Region, 2026-2033
- North America
- Europe
- East Asia
- South Asia & Oceania
- Latin America
- Middle East & Africa
- Market Attractiveness Analysis: Region
- North America Liquid-Filled Hard Capsules Market Outlook: Historical (2020 – 2025) and Forecast (2026 – 2033)
- Key Highlights
- North America Market Size (US$ Bn) Forecast, by Country, 2026-2033
- U.S.
- Canada
- North America Market Size (US$ Bn) Forecast, by Product, 2026-2033
- Liquid in Capsules
- Pellets in Liquid-filled Capsules
- Pellets in Capsule in Liquid-filled Capsules
- Capsule in Liquid-filled Capsules
- Tablet in Liquid-filled Capsules
- North America Market Size (US$ Bn) Forecast, by Raw Material, 2026-2033
- Gelatin
- Hypromellose Capsules (HPMCs)
- North America Market Size (US$ Bn) Forecast, by Application, 2026-2033
- Cough & Cold Preparations
- Cardiovascular Therapy Drugs
- Health Supplements
- Vitamin & Dietary Supplements
- Other
- North America Market Size (US$ Bn) Forecast, by End User, 2026-2033
- Pharmaceutical Companies
- Nutraceutical Companies
- Cosmeceutical Companies
- Contract Manufacturing Organizations
- Europe Liquid-Filled Hard Capsules Market Outlook: Historical (2020 – 2025) and Forecast (2026 – 2033)
- Key Highlights
- Europe Market Size (US$ Bn) Forecast, by Country, 2026-2033
- Germany
- Italy
- France
- U.K.
- Spain
- Russia
- Rest of Europe
- Europe Market Size (US$ Bn) Forecast, by Product, 2026-2033
- Liquid in Capsules
- Pellets in Liquid-filled Capsules
- Pellets in Capsule in Liquid-filled Capsules
- Capsule in Liquid-filled Capsules
- Tablet in Liquid-filled Capsules
- Europe Market Size (US$ Bn) Forecast, by Raw Material, 2026-2033
- Gelatin
- Hypromellose Capsules (HPMCs)
- Europe Market Size (US$ Bn) Forecast, by Application, 2026-2033
- Cough & Cold Preparations
- Cardiovascular Therapy Drugs
- Health Supplements
- Vitamin & Dietary Supplements
- Other
- Europe Market Size (US$ Bn) Forecast, by End User, 2026-2033
- Pharmaceutical Companies
- Nutraceutical Companies
- Cosmeceutical Companies
- Contract Manufacturing Organizations
- East Asia Liquid-Filled Hard Capsules Market Outlook: Historical (2020 – 2025) and Forecast (2026 – 2033)
- Key Highlights
- East Asia Market Size (US$ Bn) Forecast, by Country, 2026-2033
- China
- Japan
- South Korea
- East Asia Market Size (US$ Bn) Forecast, by Product, 2026-2033
- Liquid in Capsules
- Pellets in Liquid-filled Capsules
- Pellets in Capsule in Liquid-filled Capsules
- Capsule in Liquid-filled Capsules
- Tablet in Liquid-filled Capsules
- East Asia Market Size (US$ Bn) Forecast, by Raw Material, 2026-2033
- Gelatin
- Hypromellose Capsules (HPMCs)
- East Asia Market Size (US$ Bn) Forecast, by Application, 2026-2033
- Cough & Cold Preparations
- Cardiovascular Therapy Drugs
- Health Supplements
- Vitamin & Dietary Supplements
- Other
- East Asia Market Size (US$ Bn) Forecast, by End User, 2026-2033
- Pharmaceutical Companies
- Nutraceutical Companies
- Cosmeceutical Companies
- Contract Manufacturing Organizations
- South Asia & Oceania Liquid-Filled Hard Capsules Market Outlook: Historical (2020 – 2025) and Forecast (2026 – 2033)
- Key Highlights
- South Asia & Oceania Market Size (US$ Bn) Forecast, by Country, 2026-2033
- India
- Southeast Asia
- ANZ
- Rest of SAO
- South Asia & Oceania Market Size (US$ Bn) Forecast, by Product, 2026-2033
- Liquid in Capsules
- Pellets in Liquid-filled Capsules
- Pellets in Capsule in Liquid-filled Capsules
- Capsule in Liquid-filled Capsules
- Tablet in Liquid-filled Capsules
- South Asia & Oceania Market Size (US$ Bn) Forecast, by Raw Material, 2026-2033
- Gelatin
- Hypromellose Capsules (HPMCs)
- South Asia & Oceania Market Size (US$ Bn) Forecast, by Application, 2026-2033
- Cough & Cold Preparations
- Cardiovascular Therapy Drugs
- Health Supplements
- Vitamin & Dietary Supplements
- Other
- South Asia & Oceania Market Size (US$ Bn) Forecast, by End User, 2026-2033
- Pharmaceutical Companies
- Nutraceutical Companies
- Cosmeceutical Companies
- Contract Manufacturing Organizations
- Latin America Liquid-Filled Hard Capsules Market Outlook: Historical (2020 – 2025) and Forecast (2026 – 2033)
- Key Highlights
- Latin America Market Size (US$ Bn) Forecast, by Country, 2026-2033
- Brazil
- Mexico
- Rest of LATAM
- Latin America Market Size (US$ Bn) Forecast, by Product, 2026-2033
- Liquid in Capsules
- Pellets in Liquid-filled Capsules
- Pellets in Capsule in Liquid-filled Capsules
- Capsule in Liquid-filled Capsules
- Tablet in Liquid-filled Capsules
- Latin America Market Size (US$ Bn) Forecast, by Raw Material, 2026-2033
- Gelatin
- Hypromellose Capsules (HPMCs)
- Latin America Market Size (US$ Bn) Forecast, by Application, 2026-2033
- Cough & Cold Preparations
- Cardiovascular Therapy Drugs
- Health Supplements
- Vitamin & Dietary Supplements
- Other
- Latin America Market Size (US$ Bn) Forecast, by End User, 2026-2033
- Pharmaceutical Companies
- Nutraceutical Companies
- Cosmeceutical Companies
- Contract Manufacturing Organizations
- Middle East & Africa Liquid-Filled Hard Capsules Market Outlook: Historical (2020 – 2025) and Forecast (2026 – 2033)
- Key Highlights
- Middle East & Africa Market Size (US$ Bn) Forecast, by Country, 2026-2033
- GCC Countries
- South Africa
- Northern Africa
- Rest of MEA
- Middle East & Africa Market Size (US$ Bn) Forecast, by Product, 2026-2033
- Liquid in Capsules
- Pellets in Liquid-filled Capsules
- Pellets in Capsule in Liquid-filled Capsules
- Capsule in Liquid-filled Capsules
- Tablet in Liquid-filled Capsules
- Middle East & Africa Market Size (US$ Bn) Forecast, by Raw Material, 2026-2033
- Gelatin
- Hypromellose Capsules (HPMCs)
- Middle East & Africa Market Size (US$ Bn) Forecast, by Application, 2026-2033
- Cough & Cold Preparations
- Cardiovascular Therapy Drugs
- Health Supplements
- Vitamin & Dietary Supplements
- Other
- Middle East & Africa Market Size (US$ Bn) Forecast, by End User, 2026-2033
- Pharmaceutical Companies
- Nutraceutical Companies
- Cosmeceutical Companies
- Contract Manufacturing Organizations
- Competition Landscape
- Market Share Analysis, 2025
- Market Structure
- Competition Intensity Mapping
- Competition Dashboard
- Company Profiles
- Lonza
- Company Overview
- Product Portfolio/Offerings
- Key Financials
- SWOT Analysis
- Company Strategy and Key Developments
- VANTAGE NUTRITION
- INNERCAP Technologies, Inc.
- LIQUIDCAPSULE MANUFACTURING LLC
- SuHeung
- Altasciences
- Others
- Lonza
- Appendix
- Research Methodology
- Research Assumptions
- Acronyms and Abbreviations

Loading page data
Please wait a moment