ID: PMRREP14911| 201 Pages | 10 Dec 2025 | Format: PDF, Excel, PPT* | Healthcare

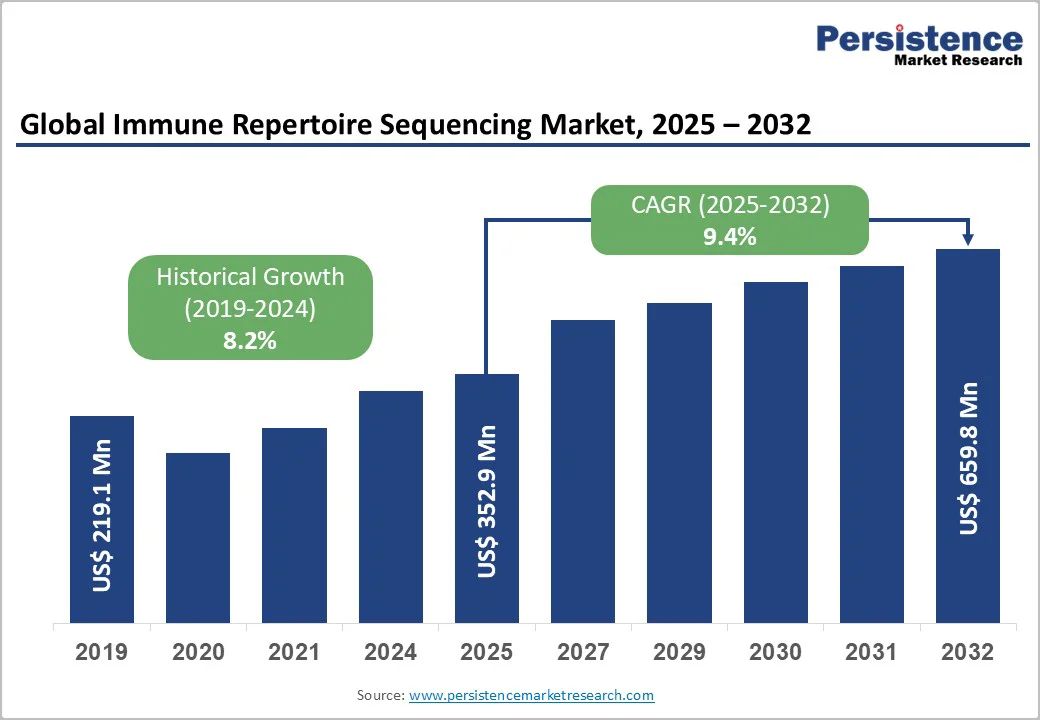
The global immune repertoire sequencing market size is valued at US$352.9 million in 2025 and is projected to reach US$659.8 million by 2032, growing at a CAGR of 9.4% between 2025 and 2032. The emphasis on precision immunology and data-driven therapeutic development is accelerating the adoption of immune repertoire sequencing worldwide.
This technology leverages next-generation sequencing to decode the full diversity of B-cell Receptor (BCR) and T-cell Receptor (TCR) repertoires, enabling highly detailed insight into how the immune system responds to infections, cancer, and vaccines.
| Key Insights | Details |
|---|---|
|
Immune Repertoire Sequencing Market Size (2025E) |
US$352.9 Million |
|
Market Value Forecast (2032F) |
US$659.8 Million |
|
Projected Growth (CAGR 2025 to 2032) |
9.4% |
|
Historical Market Growth (CAGR 2019 to 2024) |
8.2% |
The global immune repertoire sequencing market is advancing rapidly as sequencing technologies evolve and research needs become more complex. Bulk sequencing continues to play a foundational role by offering a comprehensive and cost-effective view of gene expression, supporting high-throughput analysis, target identification, and drug-response mapping. Alongside this, immune repertoire sequencing enables precise characterization of how immune cells respond to tumors, viruses, and vaccines, making it central to immunology and therapeutic development.
A major accelerator of global adoption is the work of the Adaptive Immune Receptor Repertoire (AIRR) Community of The Antibody Society. By organizing meetings, establishing AIRR data standards, and coordinating guidance for generating, analyzing, curating, and sharing AIRR-seq datasets, the community strengthens data comparability across labs and research programs. Their focus on linking AIRR-seq with broader “big data” types—such as single-cell expression, flow cytometry, and microarray outputs—further enhances scientific utility.
The market is also propelled by rapid transitions from short-read bulk T-cell Receptor Beta Chain (TCRβ)/ Immunoglobulin Heavy Chain (IGH) studies to long-read and single-cell approaches that allow researchers to trace clones, define functional states, and map trajectories across tissues and time. Initiatives such as Immunai’s Grand Collaboration Initiative are expanding access to single-cell multi-omic profiling, enriching global datasets and accelerating therapeutic discovery. Together, these shifts are reshaping the international landscape and deepening biological insight.
The adaptive immune system stores a lifelong record of pathogen encounters through highly diverse adaptive immune receptors (AIRs) expressed on B cells and T cells. These receptors carry rich biological information, positioning the AIR repertoire (AIRR) as a potential source of powerful biomarkers and therapeutic targets.
While this diversity offers immense scientific value, it also introduces major analytical challenges. The complexity and scale of AIRR datasets make it difficult to accurately decode patterns that govern antigen recognition, immune memory, or disease progression.
Although substantial efforts have focused on using machine learning (ML) to interpret AIRR data, performance remains limited for many key prediction and generation tasks, as highlighted in recent studies. High dimensionality, limited labeled datasets, and variability across individuals restrict the ability of ML models to generalize. As a result, translating AIRR-derived insights into reliable clinical tools, diagnostics, or therapeutic guidance remains slow.
These scientific and computational hurdles continue to restrain the global advancement of immune repertoire sequencing applications, underscoring the need for improved algorithms, standardized datasets, and deeper functional annotation to fully harness AIR-encoded information.
High-throughput sequencing of immunoglobulin and TCR repertoires continues to unlock major growth opportunities by expanding the boundaries of immunology, precision diagnostics, and therapeutic research. Detailed profiling of TCR CDR3 regions and complement diversity allows researchers to map adaptive immune behavior with far greater accuracy, opening possibilities in early biomarker discovery, next-generation vaccine design, and monitoring immune recovery in oncology and infectious diseases.
As immune repertoire sequencing becomes more integrated into clinical workflows, it also enables opportunities in minimal residual disease tracking, autoimmune disorder stratification, and donor–recipient matching for transplantation.
Beyond scientific gains, the field offers strong technological opportunities. The surge in sequencing output is driving demand for cloud-based analytical platforms, AI-assisted pattern recognition, and automated pipelines capable of managing full-length receptor data. These advances reduce error rates, enhance reproducibility, and support large-scale population immune-mapping initiatives that were previously infeasible.
Growing collaborations between industry, academic consortia, and computational biology groups are further accelerating innovation. As long-read and single-cell repertoire methods continue to mature, they are expected to create new clinical decision-support tools, strengthen personalized medicine strategies, and ultimately expand the global footprint of immune repertoire applications over the forecast period.
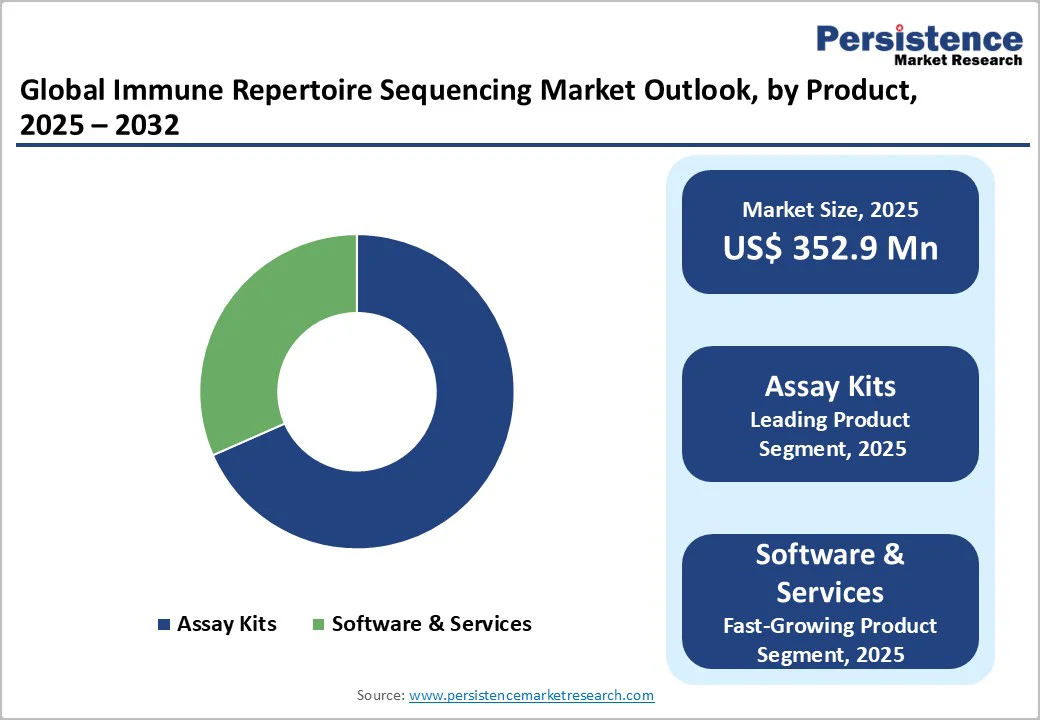
Assay kits are expected to command 68.4% of the global immune repertoire sequencing market by 2025, primarily because they offer standardized, ready-to-use formats that reduce technical variability and streamline complex sequencing workflows. Their ability to deliver consistent, reproducible results across labs makes them the preferred choice for high-throughput studies.
Additionally, increasing adoption of targeted immune profiling in oncology, vaccine research, and autoimmune disease studies continues to boost demand. As researchers prioritize accuracy and rapid turnaround, assay kits remain the most dependable and scalable product category in this space.
Drug discovery and development is projected to capture 38.4% of the global market in 2025, driven by growing reliance on immune repertoire sequencing understanding therapeutic mechanisms, predicting patient response, and identifying novel drug targets.
As immunotherapies, cell therapies, and vaccine platforms expand, researchers increasingly use repertoire profiling to decode antigen-specific signatures and guide candidate selection. This application also benefits from integration with single-cell analysis and multi-omic tools, enabling deeper characterization of immune dynamics. Collectively, these capabilities position drug discovery as the most influential application area.
Pharma and biotech companies are projected to dominate the market in 2025, capturing 38.4% of the total share, supported by their extensive R&D programs and rapid adoption of advanced sequencing technologies. These companies increasingly rely on immune repertoire analysis to accelerate therapeutic pipelines, especially in oncology, infectious diseases, and autoimmune disorders.
Their ability to invest in high-throughput platforms, automation, and computational tools gives them a competitive edge. Furthermore, expanding collaborations with academic institutes and technology providers enhances translational research, reinforcing their position as the primary end-user segment.
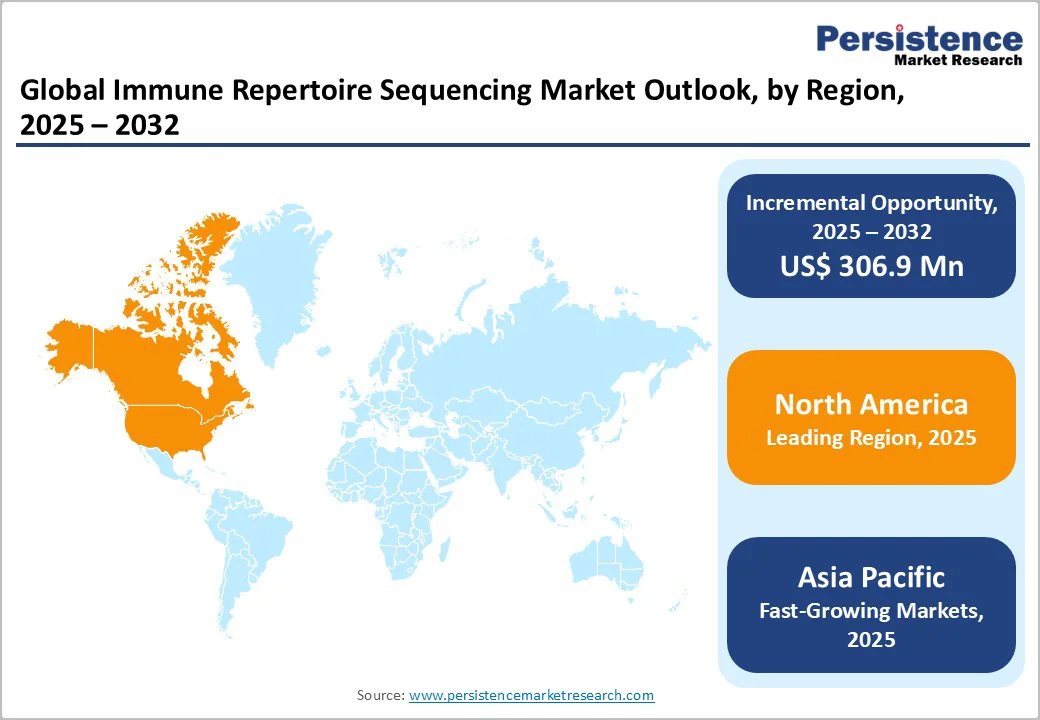
In 2025, North America accounted for nearly 40.2% of the global immune repertoire sequencing market, supported by its extensive genomics ecosystem and high-throughput NGS infrastructure. The region continues to lead due to its large volume of clinical trials that increasingly incorporate repertoire profiling to understand immune responses, optimize treatment pathways, and evaluate emerging immunotherapies. This momentum aligns with the growing cancer burden in the United States.
According to the U.S. National Cancer Institute, an estimated 2,041,910 new cancer cases and 618,120 deaths are expected in 2025, underscoring the urgent need for deeper immunological insights. As recent scientific literature highlights, progress against cancer now relies heavily on immune-based therapies and next-generation vaccine development, which depend on precise mapping of T-cell and B-cell receptor diversity. Immune repertoire sequencing directly enables this by revealing clonal evolution, tracking treatment response, and guiding personalized therapeutic strategies. Together, these scientific, clinical, and infrastructural strengths reinforce North America’s dominant position, making it a key driver of innovation and adoption in immune repertoire sequencing through 2025 and beyond.
By 2025, Europe is expected to capture nearly 28.4% of the global immune repertoire sequencing market, supported by its strong push toward personalized medicine and biomarker-driven research programs.
The region has emerged as the second-largest market as rising healthcare expenditure and sustained government funding continue to accelerate research in genomics, autoimmune diseases, and oncology—areas where immune repertoire sequencing plays a critical role in understanding immune diversity and disease progression.
Europe’s position is further strengthened by the presence of strong academic consortia and translational research centers, which actively collaborate across institutions to advance immune-profiling technologies. This ecosystem, combined with the concentration of several regional manufacturers, has created a progressively competitive market landscape.
Such competition, along with substantial capital investments in biomedical research, has contributed to a steady decline in sequencing costs, making high-resolution repertoire analysis more accessible to research and clinical laboratories.
Overall, Europe’s mix of research intensity, funding support, and a well-established innovation network continues to drive adoption, reinforcing its role as a key hub for immune repertoire sequencing advancements by 2025.
The Asia Pacific immune repertoire sequencing market is poised for rapid growth, projected to expand at a CAGR of 11.8% over the forecast period. This expansion is largely driven by a swift build-out of biotech and sequencing capacity across the region, particularly in China and Japan, where investments in genomics infrastructure are scaling quickly. Rising healthcare spending and a growing cancer burden further fuel demand for advanced immune profiling.
Adoption of immune repertoire sequencing has increased sharply in Asia Pacific, as researchers and clinicians leverage this technology for immunology, oncology, and precision medicine. Moreover, international collaborations and partnerships—especially involving China and Japan—are accelerating the integration of next-generation immune sequencing technologies.
Together, these factors form a powerful ecosystem: robust R&D capability, growing demand, funding support, and global cooperation, all of which are expected to drive strong immune repertoire sequencing market growth in the Asia Pacific through the forecast period.
The competitive landscape is defined by rapid advances in immune-repertoire sequencing, single-cell analysis, and T-cell–targeted therapeutics. Players are expanding platforms for high-resolution BCR/TCR profiling, automating complex NGS workflows, and pursuing strategic collaborations to accelerate autoimmune therapy development.
Innovations in paired-chain sequencing, tolerizing vaccine design, and unbiased V(D)J analysis are driving differentiation, with companies competing on scalability, sensitivity, and integration of discovery-to-clinical monitoring capabilities.
The global immune repertoire sequencing market is projected to be valued at US$ 352.9 Million in 2025.
Advancements in NGS technologies, rising immunotherapy research, and growing demand for high-resolution immune profiling drive global immune repertoire sequencing adoption.
The global market is poised to witness a CAGR of 9.4% between 2025 and 2032.
Expanding single-cell sequencing, AI-driven analytics, and applications in precision medicine, vaccine development, and biomarker discovery offer strong future growth opportunities.
Major players in the global are Illumina, Inc., PacBio, Agilent Technologies, Inc., Oxford Nanopore Technologies plc, QIAGEN, Thermo Fisher Scientific Inc., F. Hoffmann-La Roche Ltd., and others.
| Report Attribute | Details |
|---|---|
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis |
Value: US$ Mn |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
By Product
By Application
By End-user
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author