ID: PMRREP22714| 191 Pages | 6 Nov 2025 | Format: PDF, Excel, PPT* | Healthcare

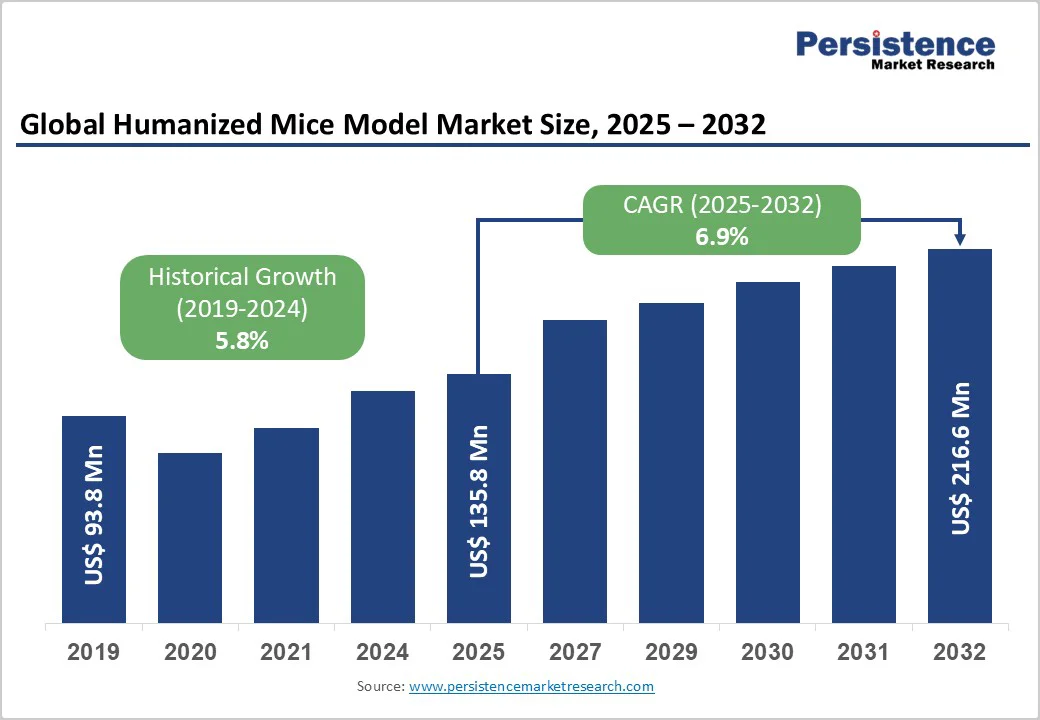
The global humanized mice model market size is likely to value US$ 135.8 million in 2025 and is projected to reach US$ 216.6 million growing at a CAGR of 6.9% during the forecast period from 2025 to 2032. The global humanized mice model market is growing steadily, driven by increasing demand for preclinical studies, rising focus on personalized medicine, and advancements in immunodeficient mouse models. North America dominates due to advanced research infrastructure and a strong biotech presence, while Asia Pacific is the fastest-growing region, driven by expanding biomedical research, rising R&D funding, and supportive government initiatives.
| Key Insights | Details |
|---|---|
|
Global Humanized Mice Model Market Size (2025E) |
US$ 135.8 Mn |
|
Market Value Forecast (2032F) |
US$ 216.6 Mn |
|
Projected Growth (CAGR 2025 to 2032) |
6.9% |
|
Historical Market Growth (CAGR 2019 to 2024) |
5.8% |
The rising demand for oncology and immunology research is a major driver of the Humanized Mice Model Market. Globally, cancer remains a leading cause of mortality, with an estimated 20 million new cases and 9.7 million deaths in 2022, according to the World Health Organization (WHO). This rising disease burden has accelerated the need for preclinical models that closely mimic human biological systems to evaluate new cancer drugs, immunotherapies, and targeted therapies.
In parallel, the field of immunology is witnessing substantial growth, fueled by the increasing prevalence of autoimmune and infectious diseases and expanding research in immune-based treatments. The U.S. National Institute of Allergy and Infectious Diseases (NIAID) allocated approximately US$6.56 billion in FY 2023 for immunology and infectious disease research. These growing investments and disease trends have intensified the adoption of humanized mice, which enable accurate in-vivo assessment of human immune responses, thereby advancing oncology and immunology breakthroughs.
One significant restraint in the humanized mice model market is the technical challenges of humanization, particularly achieving consistent, high-quality engraftment of human cells. For example, studies show that in one series using the NSG mouse strain engrafted with human CD34+ hematopoietic stem cells (HSCs), only 48.5 ± 21 % chimerism was achieved after eight weeks (n = 26) and 41.6 ± 19 % (n = 15), indicates wide variability.
Moreover, donor-to-donor and mouse-to-mouse variability remains a problem: one melanoma PDX humanisation study noted that even mice engrafted from the same donor exhibited radically different cell-lineage distributions. Compounding this, some humanised strains (e.g., NSG-SGM3) showed severely reduced lifespans, and all mice died between 10 and 27 weeks post-engraftment owing to graft-versus-host-like effects. These technical inconsistencies undermine reproducibility, increase cost, and limit standardisation of preclinical pipelines, thus restraining market uptake and scalability.
Development of next-generation transgenic and multi-lineage humanized mouse models presents a strategic opportunity for preclinical research and drug discovery. Government agencies have signaled support through funding notices to develop and improve animal and tumor models (e.g., NIH PAR-25-273, NCI PAR-24-306 issued in 2024). Concomitantly, gene-editing advances such as CRISPR have matured rapidly, with bibliometric studies documenting hundreds of CRISPR-related publications from 2015–2022, reflecting broad methodological adoption. Clinical translation of gene editing (for example, the FDA-cleared CRISPR-based therapy exa-cel) further validates investment in precise in-vivo platforms that recapitulate human biology. NIH and NIAID policies encouraging model sharing and standardized resources reduce duplication and facilitate broader access to sophisticated strains. Taken together, clear funding pathways, accelerating CRISPR-enabled engineering, and stronger sharing frameworks lower technical barriers, shorten development timelines, and make next-generation transgenic, multi-lineage humanized models an attractive, high-impact opportunity for biopharma and academic investors and improve translational predictability across therapeutic areas.
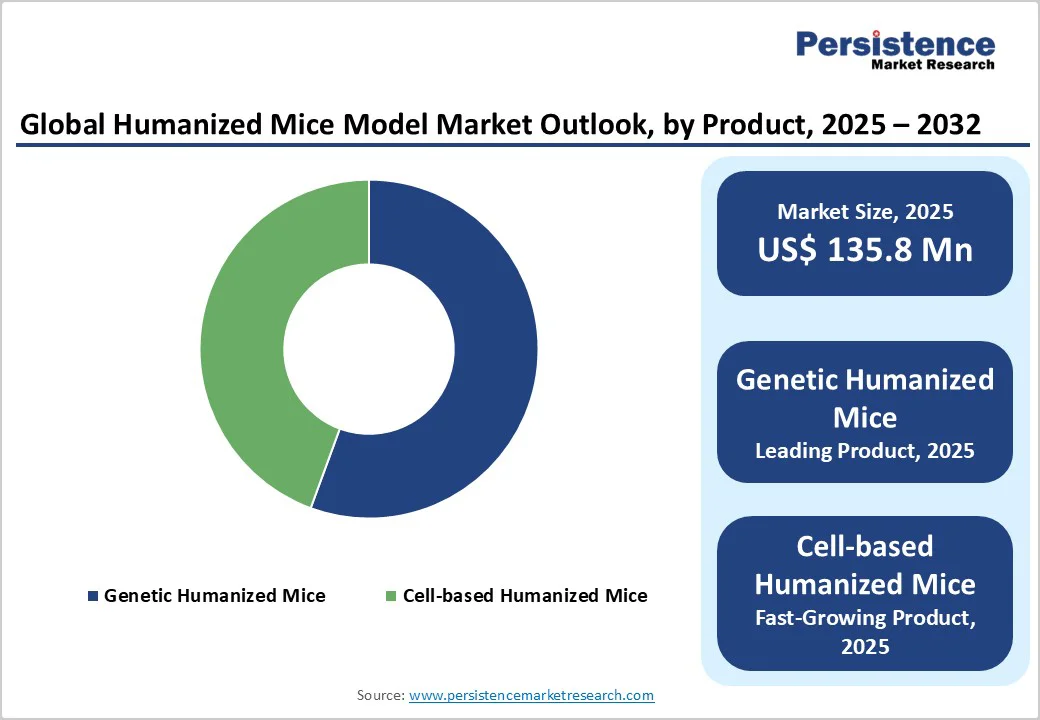
The genetically humanized mice is dominant with 55.6% share in 2025, due to their advanced ability to replicate human gene expression and immune responses. These models are created by inserting specific human genes into mouse genomes, allowing researchers to study complex biological mechanisms, immune reactions, and disease progression more accurately. According to the U.S. National Institutes of Health (NIH), the adoption of genetically engineered mice has significantly increased in cancer, infectious disease, and immunology research, as they enable precise modeling of human-specific pathways. Continuous advancements in CRISPR-Cas9 and transgenic technologies have improved gene targeting efficiency, boosting reliability and scalability. As a result, genetically humanized mice are becoming the preferred choice for drug discovery and preclinical validation studies worldwide.
The dominance of Oncology in the market driven by the global rise in cancer incidence and the growing need for effective preclinical models. According to the World Health Organization (WHO), cancer caused nearly 10 million deaths in 2022, making it one of the leading causes of mortality worldwide. Humanized mice enable researchers to study tumor growth, immune evasion, and responses to immunotherapies in a system closely resembling human physiology. These models are extensively used in testing immune checkpoint inhibitors, CAR-T cell therapies, and targeted biologics. Their ability to replicate the human tumor microenvironment and predict clinical outcomes has made them indispensable in oncology research, supporting faster drug development and improved translational accuracy.
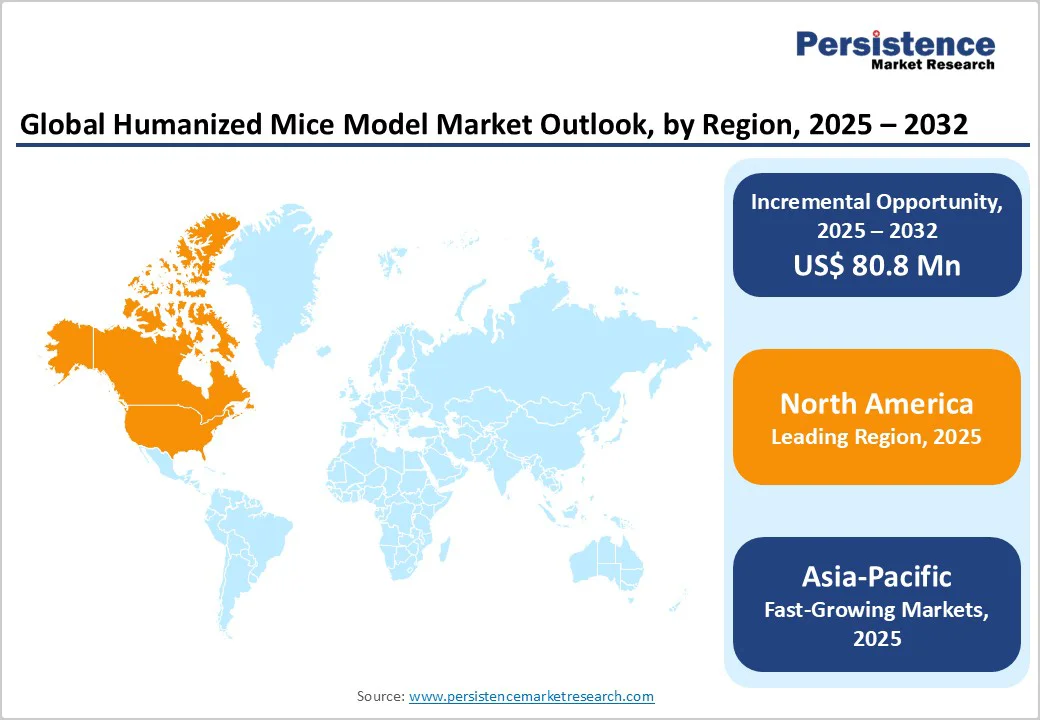
North America region dominates the global market with a 45.2% share in 2025, due to its advanced biomedical research infrastructure, strong presence of pharmaceutical and biotechnology companies, and high R&D investment levels. The U.S. National Institutes of Health (NIH) allocated over US$47 billion in 2024 for medical research, with significant portions directed toward cancer, immunology, and genetic studies, key areas using humanized mice.
Additionally, the region benefits from well-established contract research organizations (CROs), supportive regulatory frameworks, and access to cutting-edge technologies like CRISPR and stem cell engineering. Academic institutions and research centers across the U.S. and Canada actively collaborate with industry partners, further driving innovation. These factors collectively position North America as the dominant hub for humanized mouse model development and commercialization.
Europe represents a key region in the market due to its strong network of biomedical research institutions, government funding, and emphasis on translational medicine. The European Commission’s Horizon Europe program, with a budget of €95.5 billion (2021–2027), supports extensive research in genomics, oncology, and immunology fields that heavily rely on humanized models. Countries like Germany, the U.K., and France host major contract research organizations (CROs) and academic collaborations focused on preclinical drug development. Additionally, Europe’s advanced regulatory environment under the European Medicines Agency (EMA) ensures ethical and standardized animal research practices. The growing focus on personalized medicine and immuno-oncology further strengthens Europe’s position as a major hub for humanized mouse model adoption.
Asia Pacific is the fastest-growing region in the Humanized Mice Model Market, driven by rapid expansion in biomedical research, rising healthcare expenditure, and increasing government support for translational studies. Countries like China, Japan, South Korea, and India are investing heavily in genomics, oncology, and immunology research to strengthen domestic drug discovery capabilities. For example, China’s 14th Five-Year Plan emphasizes biotechnology innovation and preclinical infrastructure development. Growing collaborations between pharmaceutical companies and academic institutes, along with expanding contract research organizations (CROs), are accelerating the use of advanced humanized models. Additionally, improvements in laboratory infrastructure, adoption of CRISPR-based technologies, and rising demand for personalized medicine are propelling Asia Pacific’s rapid market growth and global competitiveness in biomedical research.
Leading companies in the humanized mice model market are focusing on developing advanced and customizable mouse models, securing regulatory approvals, and forming strategic collaborations with pharmaceutical and biotechnology firms. Key players are investing in next-generation CD34+ and genetic humanized mice, integrating CRISPR and stem cell technologies to enhance accuracy and translational relevance. Several companies are expanding their global footprint through partnerships, mergers, and acquisitions to strengthen research capabilities and service portfolios. These initiatives aim to accelerate drug discovery, immuno-oncology research, and personalized medicine development, while ensuring higher reproducibility and ethical compliance. Continuous innovation in model design and human immune system reconstitution remains central to meeting the growing demand for reliable preclinical research tools worldwide.
The global humanized mice model market is projected to be valued at US$ 135.8 Mn in 2025.
Rising demand for oncology research, personalized medicine, and immunotherapy, supported by advancements in genetic engineering and increased R&D investments.
The global humanized mice model market is poised to witness a CAGR of 6.9% between 2025 and 2032.
Expansion in immuno-oncology, CRISPR-based model development, AI integration, and growing collaborations between pharma, CROs, and academic research institutions.
Taconic Biosciences, Biocytogen, Charles River Laboratories, The Jackson Laboratory (JAX), genOway, Champions Oncology.
| Report Attribute | Details |
|---|---|
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis |
Value: US$ Mn |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
By Model Type
By Application
By End-user
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author