ID: PMRREP22879| Upcoming | Format: PDF, Excel, PPT* | Healthcare

With the increasingly stressful lifestyle there is also rise in other types of disorders such as anxiety and sleep disorders. With the lack of sufficient treatments that address these disorders, cranial electrotherapy stimulation comes as an innovative and required treatment.
Cranial Electrotherapy Stimulation (CES) device is a small device that stimulates the cranium and brain with a current less than 4 mA, which cannot be sensed by the patient. The cranial electrotherapy stimulation is approved by the FDA for the treatment of insomnia, depression and anxiety.
Besides, it has potential application in the treatment of a number of disorders such as attention deficit hyperactivity disorder (ADHA), obsessive-compulsive disorder, post-traumatic stress disorder (PTSD), cognitive dysfunction, traumatic brain injury, pain, enhancing attention and concentration, and for reducing assaultive behavior.
A relatively large number of the population across the globe is diagnosed with such disorders. A significant portion of the U.S. population is affected by poor mental health, which leads to development of various kinds of mental health disorders. The treatment method is complementary and an alternative of medicine.
There are large number of clinical trials currently active, which have proved the CES device as an effective treatment method. For an instance, as of 2017 the Sham Cranial Electrotherapy Stimulation by Electromedical Products International Inc. is under clinical trial and is currently recruiting candidates for the same.
The increasing number of cases of poor mental health with the development of disorders such as depression, anxiety and other sleep disorders is driving the growth of the market. Although animal studies have proved this device to be effective, the adoption of these devices is affected due to lack of strong evidence in humans proving the efficacy of the devices in all or most of the cases.
However, there are a number of new clinical trials in the recruiting stage, which may help fill the gap in the market.
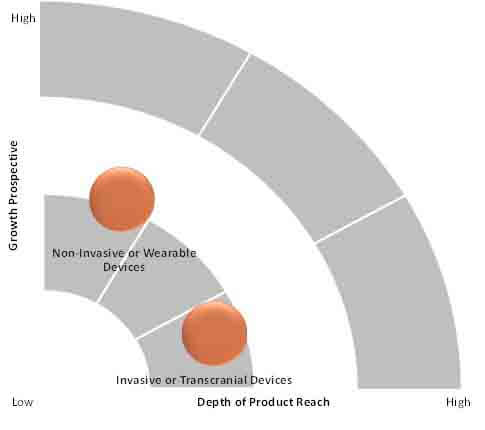
There are various types of cranial electrotherapy stimulator devices and they can be broadly classified based on the type of electrode placement, such as invasive or transcranial and non-invasive or wearable. The wearable type is the dominant segment in the market.
The wearable type device is user-friendly and does not requires surgical insertion of the electrode. Most of the FDA-approved devices are suitable for the treatment of insomnia, depression and anxiety, as the prevalence of these disorders is increasing, in which depression is the most common and growing disorder in the young population.
Since most of these devices are available only in prescribed hospitals and mental health clinics, they have a large scope as end-use segments in the market.
North America has a large number of mental health cases, which has increased the use of the device in the region. Also, growing awareness regarding the treatment of mental health along with technological advancements make North America a potential market for electrotherapy stimulation devices.
Likewise, growing awareness in other regions such as Asia Pacific, there is a scope for rapid development in the region.
There are three major cranial electrotherapy stimulation devices, namely the CES Ultra by Neuro-fitnesss LLC, Alpha-Stim M and the Alpha-Stim AID by Electromedical Products International (EPI), and the Fisher-Wallace Simulator. Other FDA-approved products include BR-2 Biorest (Biorest Inc), Biotron 18 (Biotronics Corp), Elexoma Medic (Redplane AG), FM 10/C (Johari Digital Healthcare Ltd.), and HP-1 Heathpax or Nurtipax (Health Directions Inc), among others.
Examples of some of the key players in the global cranial electrotherapy stimulation devices are as follows:
| Small-Scale Manufacturers/Providers |
|
|
Medium-Scale Manufacturers/Providers |
|
|
Large-Scale Manufacturers/Providers |
|
The report covers exhaustive analysis on:
PMR utilizes a triangulation methodology that is primarily based on experimental techniques such as patient-level data, number of procedures and capital equipment install base to obtain precise market estimations and insights on various medical devices and medical technology.
Bottom-up approach is always used to obtain insightful data for the specific country/regions. The country-specific data is again analyzed to derive data at a global level. This methodology ensures high quality and accuracy of information.
Secondary research is used at the initial phase to identify the feasibility of the target products/technology categories and its respective segments, product offerings, usage pattern as per disease indications, product installed base in target healthcare facilities, life span of a device, reimbursement scenario, adoption rate and future impact of new technologies.
Each piece of information is eventually analyzed during the entire research project, which builds a strong base for the primary research information.
Primary research participants include demand-side users such as key opinion leaders, physicians, surgeons, and supply-side providers of medical devices who provide valuable insights on trends, key treatment patterns, adoption rate, and purchasing pattern, technological development of medical devices, patient education, effectiveness of manufacturers and important strategies, pricing and competitive dynamics.
Quantitative and qualitative assessment of basic factors driving demand, economic factors/cycles and growth rates and strategies utilized by key players in the market are analyzed in detail while forecasting, in order to project Year-on-Year growth rates. These Y-o-Y growth projections are checked and aligned as per industry/product lifecycle and further utilized to develop market numbers at a holistic level.
On the other hand, we also analyze various annual reports_bk_01_01_2020 of different companies, investor presentations, SEC filings, 10k reports_bk_01_01_2020 and press release operating in this market segment to fetch substantial information about the market size, trends, opportunity, drivers, and restraints to analyze key players and their market shares. Key companies are segmented at Tier level based on their revenues, product portfolio and presence.
Please note that these are the partial steps that are being followed while developing the market size. Besides this, forecasting will be done based on our internal proprietary model which also uses different macro-economic factors such as per capita healthcare expenditure, disposable income, industry based demand driving factors impacting the market and its forecast trends apart from disease related factors.
| By Method Type |
|
| By End User |
|
|
Region |
|
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author