ID: PMRREP33710| 199 Pages | 2 Jun 2025 | Format: PDF, Excel, PPT* | Healthcare

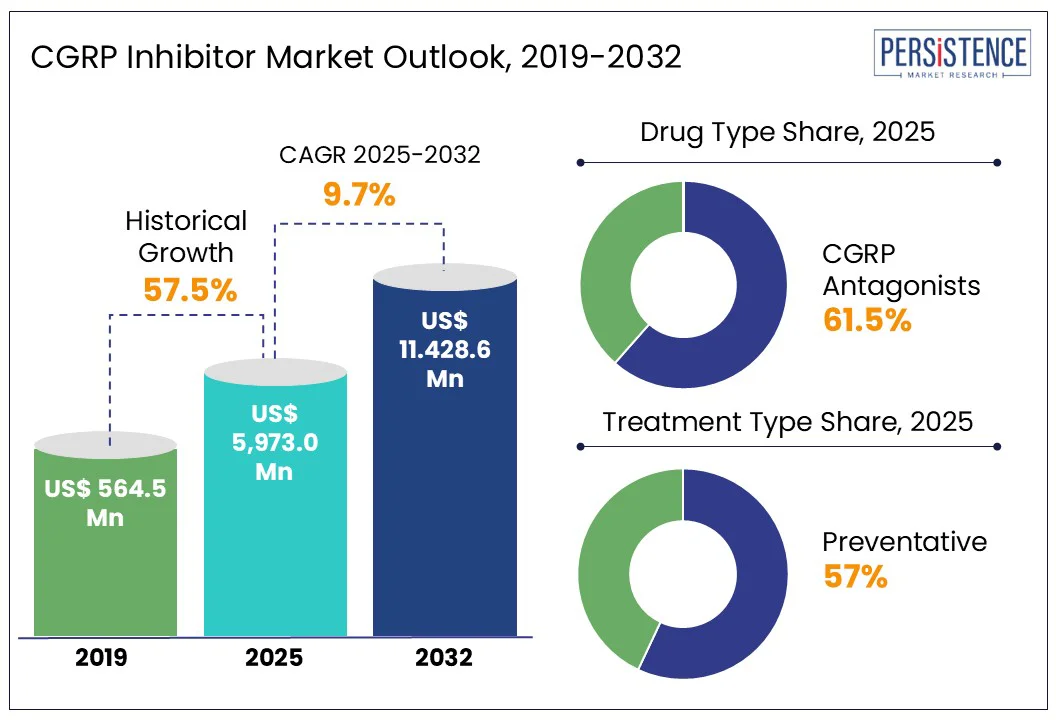
The global CGRP Inhibitors Market size is anticipated to reach a value of US$ 5,973.0 Mn in 2025 and is set to witness a CAGR of 9.7% from 2025 to 2032. The market will likely attain a value of US$ 11,428.6 Mn in 2032. According to the Persistence Market Research report, CGRP inhibitors have transformed migraine treatment since their introduction, offering targeted therapy with fewer side effects compared to traditional options such as triptans or antiepileptics. Initially approved for adults with episodic and chronic migraines, drugs such as erenumab, fremanezumab, and galcanezumab demonstrated strong efficacy in reducing migraine frequency and improving quality of life. However, high costs and insurance limitations have restricted access for many patients.
Key Industry Highlights:
|
Global Market Attribute |
Key Insights |
|
CGRP Inhibitors Market Size (2025E) |
US$ 5,973.0 Mn |
|
Market Value Forecast (2032F) |
US$ 11,428.6 Mn |
|
Projected Growth (CAGR 2025 to 2032) |
9.7% |
|
Historical Market Growth (CAGR 2019 to 2024) |
57.5% |
Innovative drug delivery technologies have improved patient adherence, convenience, and treatment outcomes. Traditional migraine treatments often required injections or multiple daily doses, which posed barriers for many patients. However, newer CGRP receptor antagonists, such as rimegepant and ubrogepant, have been formulated as orally disintegrating tablets (ODTs), allowing for easier administration without water.
This is particularly beneficial for migraine sufferers, who often experience nausea and vomiting during attacks, symptoms that can make swallowing pills difficult. The effectiveness and convenience of these ODT formulations are evident in their growing adoption.
For example, rimegepant, marketed as Nurtec® ODT, generated over US$435 million in U.S. sales, reflecting strong market acceptance. Additionally, Biohaven reported that more than one million prescriptions of Nurtec® ODT were filled within the first 18 months of launch, indicating high demand driven by its fast onset and ease of use. These technologies widely enhance patient experience and also expand access to treatment for those hesitant to use injectables, thus fueling the overall CGRP inhibitor market growth.
Cost and accessibility remain major restraints in the CGRP inhibitor market, limiting widespread adoption despite clinical effectiveness. Injectable monoclonal antibodies such as erenumab and fremanezumab often cost over US$500–US$600 monthly without insurance, making them unaffordable for many especially in low- and middle-income countries. Even in developed nations, insurance gaps, high co-pays, and prior authorization requirements create access barriers. A 2022 Migraine.com article highlighted patient struggles with insurance denials and affordability. In emerging markets, CGRP inhibitors are often excluded from public health formularies, forcing reliance on out-of-pocket spending or less effective alternatives. The absence of biosimilars worsens the issue. Until affordable options or broader coverage emerges, these challenges will hinder market growth and equity.
The CGRP inhibitor market is seeing rising interest in combination therapies to enhance efficacy, especially in patients with partial or no response to monotherapy. Chronic migraine sufferers often need multi-modal approaches, leading to combinations of CGRP inhibitors with botulinum toxin (Botox), anti-epileptics, triptans, or behavioral therapies.
A 2022 Cephalalgia study found that adding onabotulinumtoxinA to a CGRP monoclonal antibody reduced migraine days by 2–3 more per month than CGRP monotherapy alone. These additive effects open opportunities for more personalized treatments targeting multiple migraine pathways. Combination approaches also support expanded labeling and lifecycle strategies, encouraging clinical trials and increasing the addressable market. As focus shifts toward real-world effectiveness and patient-centered outcomes, combination therapies represent a key next step in unlocking the full clinical potential of CGRP inhibitors.
The CGRP inhibitor market is largely propelled by the growing demand for CGRP antagonists, particularly oral options like rimegepant and ubrogepant. These medications are favored for their ease of use and rapid pain relief, making them a preferred choice over traditional treatments that often come with more side effects. This increasing patient preference has allowed oral CGRP inhibitors to capture a significant portion of the market.
At the same time, monoclonal antibodies such as erenumab, fremanezumab, and galcanezumab contribute significantly to the landscape, primarily used for migraine prevention. Administered via injection, they effectively reduce monthly migraine days and are generally well-tolerated. While they may be costlier and less convenient than oral drugs, they offer a strong preventive solution for those with frequent or severe migraines. Together, oral antagonists and injectable monoclonal antibodies are redefining the future of migraine care.
The preventive treatment segment leads the CGRP inhibitor market due to strong clinical outcomes and patient adherence. Monoclonal antibodies such as erenumab and fremanezumab have shown significant reductions in monthly migraine days. A meta-analysis of 11 trials (4,402 patients) found a mean reduction of 1.44 days and a 50% responder rate higher than placebo (RR 1.51). Real-world studies also confirm long-term effectiveness. The American Headache Society recommends CGRP inhibitors as first-line preventive therapies. Moreover, data show reduced use of traditional preventives like topiramate since CGRP mAbs became available. These factors make preventive CGRP therapies the preferred choice in migraine management.
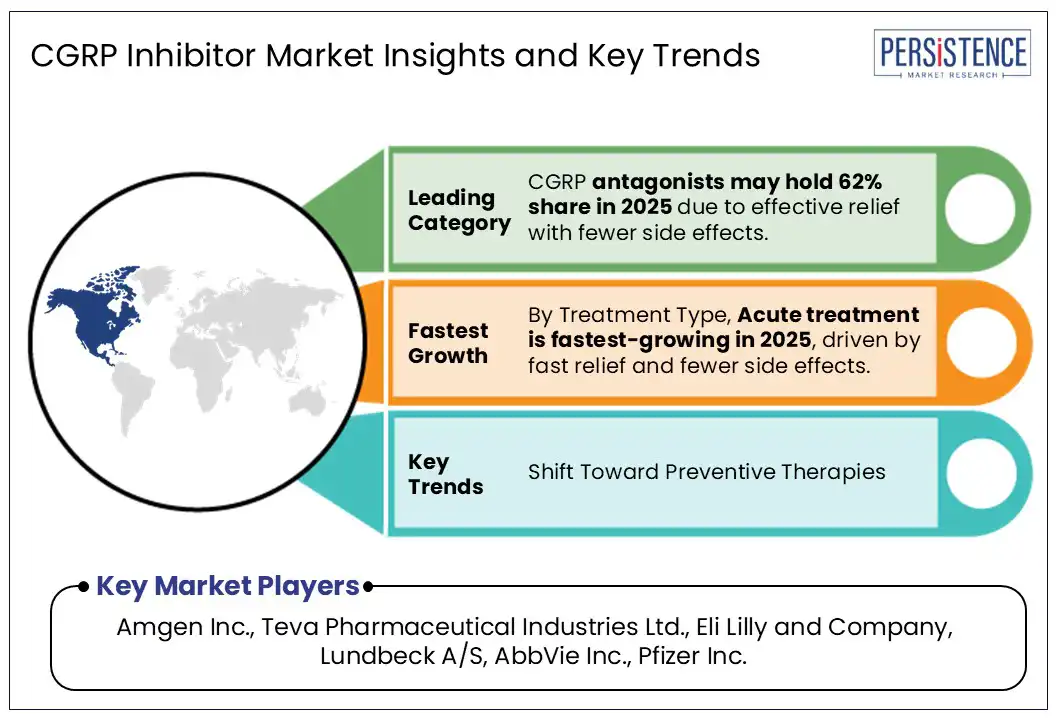
North America accounts for the largest share of the CGRP inhibitor market, driven by a high prevalence of migraine diagnoses, heightened awareness of advanced treatment options, and a robust healthcare system. In the U.S., access to specialists and innovative therapies has supported widespread use of CGRP inhibitors such as erenumab, fremanezumab, and rimegepant. While insurance hurdles exist, coverage is generally more comprehensive than in other regions, enhancing treatment accessibility.
Additionally, pharmaceutical companies prefer North America for clinical trials and product launches, in the pursuit of fast regulatory approvals. Ongoing efforts to expand insurance access and include younger patient groups, such as adolescents, are likely to drive demand. With a strong presence of major industry players and supportive infrastructure, North America is positioned to maintain its lead in the CGRP inhibitor market for the foreseeable future.
Europe is among the fastest-growing regions in the CGRP inhibitor market, primarily due to a high prevalence of migraines and the significant economic burden they impose. Approximately 41 million adults in Europe suffer from migraines, with 30.5 million having physician-diagnosed migraines in major countries such as Germany, France, the UK, Italy, and Spain, reflecting a prevalence of about 11.5%. Migraines cost Europe over €50 billion annually through healthcare expenses and lost productivity.
The European Medicines Agency (EMA) has approved several CGRP inhibitors, improving patient access to advanced therapies. Enhanced healthcare infrastructure, better insurance coverage, and growing awareness of innovative treatments contribute to increasing adoption. These factors collectively fuel rapid growth in the CGRP inhibitor market across Europe, with ongoing clinical developments and regulatory support expected to sustain momentum in the coming years.
Asia Pacific is expected to witness the fast compound annual growth rate (CAGR) in the CGRP inhibitors market during the forecast period. This surge is primarily attributed to the rising incidence of migraines, influenced by cultural, lifestyle, and environmental stressors such as poor sleep, emotional strain, and dietary habits. As awareness grows and the need for effective migraine therapies increases, CGRP inhibitors are emerging as a crucial solution.
A January 2024 study found that in India, migraine triggers included emotional stress (97.5%), weather changes (69%), physical exertion (64.5%), missed meals (63.5%), travel (55.5%), and sleep deprivation (55%). These widespread triggers are fueling demand for advanced treatment options, making APAC a significant growth region for CGRP inhibitor adoption.
The global CGRP inhibitors market is highly competitive, dominated by key players such as Amgen, Eli Lilly, and Teva Pharmaceuticals. These companies focus on innovation, strategic partnerships, and expanding product pipelines. Continuous clinical trials and regulatory approvals drive market positioning, while efforts to improve drug accessibility and affordability enhance competitive advantage.
Key Industry Developments
The global market is estimated to increase from US$ 5,973.0 Mn in 2025 to US$ 11,428.6 Mn in 2032.
Rising migraine prevalence, effective oral and injectable therapies, growing awareness, improved healthcare access, and expanding clinical approvals drive the CGRP inhibitors market.
The market is projected to record a CAGR of 9.7% during the forecast period from 2025 to 2032.
Opportunities include combination therapies, expanding insurance coverage, pediatric approvals, emerging markets growth, and the development of affordable biosimilars.
Major players include Amgen Inc., Teva Pharmaceutical Industries Ltd., Eli Lilly and Company, Lundbeck A/S, AbbVie Inc., Pfizer Inc. and Others.
|
Report Attribute |
Details |
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis Units |
Value: US$ Mn, Volume: As applicable |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
|
Customization and Pricing |
Available upon request |
By Material
By Product Type
By Route of Administration
By Distribution Channel
By Patient Demographics
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author