ID: PMRREP14572| 179 Pages | 26 Mar 2025 | Format: PDF, Excel, PPT* | Healthcare

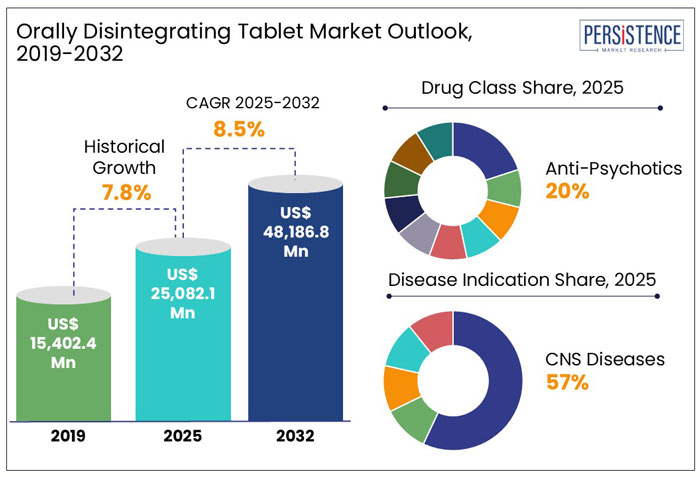
The global orally disintegrating tablet market size is anticipated to reach a value of US$ 25,082.1 Mn in 2025 and is set to witness a CAGR of 8.5% from 2025 to 2032. The market will likely attain a value of US$ 48,186.8 Mn in 2032.
The oral route of drug administration is the usual approach in the pharmaceutical business since it is the most cost-effective, easier, and safer way of drug administration. The tablets are normally taken with water.
The oral tablets enter the gastrointestinal tract and are immediately absorbed into the bloodstream. However, the orally disintegrating tablets are solid dosage forms that quickly dissolve in the mouth and do not require water to ingest. Orally disintegrating tablets are increasingly being used as an alternative to traditional tablets or capsules.
Key Highlights of the Orally Disintegrating Tablet Industry
|
Global Market Attributes |
Key Insights |
|
Orally Disintegrating Tablet Market Size (2025E) |
US$ 25,082.1 Mn |
|
Market Value Forecast (2032F) |
US$ 48,186.8 Mn |
|
Projected Growth (CAGR 2025 to 2032) |
8.5% |
|
Historical Market Growth (CAGR 2019 to 2024) |
7.8% |
In the historical period from 2019 to 2024, the global orally disintegrating tablet industry witnessed a CAGR of 7.8%.
The market is likely to have a CAGR of 8.5% over the forecast period. One of the primary factors driving the growth of Orally Disintegrating Tablets is that these tablets are increasingly being used as an alternative to traditional tablets or capsules. Apart from this, the rising adoption of technologically improved products and an increase in the prevalence of CNS illnesses such as depression, migraine, Parkinson's disease, Alzheimer's disease, and schizophrenia are likely to drive market growth.
Increased Adoption of Orally Disintegrating Tablet
Because of improved patient compliance, orally disintegrating tablets (ODTs) are being increasingly adopted over the last few years as a preferable substitute for traditional tablets and capsules. Orally disintegrating tablets dissolve in the mouth within minutes and do not require water to ingest. Traditional medications are difficult to swallow for infants, young children, and elderly people. To address such complications, various orally disintegrating pills with increased performance are developed and launched. Furthermore, advancements like enhanced taste and disintegration are enhancing ODT adoption.
Limited drug load capacity
One of the significant restraints in the growth of the orally disintegrating tablet (ODT) market is the limited drug load capacity. ODTs are specifically designed to disintegrate quickly in the mouth without the need for water, which requires them to be smaller in size and formulated with rapidly dissolving excipients. However, this structural constraint poses a challenge when incorporating higher doses of active pharmaceutical ingredients (APIs). As a result, ODTs are typically restricted to medications with lower dosage requirements, limiting their applicability for drugs that necessitate higher concentrations for therapeutic efficacy.
For instance, Adare Pharma Solutions’ Advatabs technology supports drug loading of up to 500 mg, which, while higher than many traditional ODTs, still falls short for certain high-dose medications.
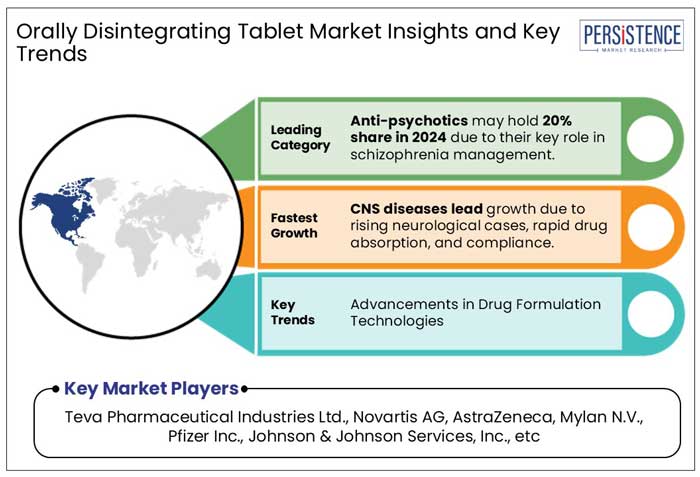
Market Dominance of Anti-Psychotic Drugs in the Orally Disintegrating Tablet (ODT) Segment
Anti-psychotic drugs are leading the drug class category in the orally disintegrating tablet (ODT) market projected to hold 19.7% of market share in 2025, due to their crucial role in managing schizophrenia, bipolar disorder, and other psychiatric conditions where patient compliance is a major concern. Many patients with these disorders struggle with swallowing pills or may be non-adherent to treatment, making ODTs a preferred option as they dissolve quickly without water. Additionally, the rapid onset of action in ODT formulations is beneficial for managing acute psychiatric episodes. Major pharmaceutical companies have also focused on developing ODT versions of widely prescribed antipsychotics, further driving market growth.
CNS Disorders Driving Growth in the Orally Disintegrating Tablet (ODT) Market
Central Nervous System (CNS) diseases dominate the market and is projected to hold 56.7% of market share in 2025, over gastrointestinal (GI) diseases, cardiovascular system (CVS) disorders, allergies, and other Disease Indication. This is primarily due to the high prevalence of neurological conditions like epilepsy, Parkinson's disease, and migraines, which often require rapid-onset medications for acute symptom management. ODTs offer a convenient administration route for patients with dysphagia or difficulty swallowing, common among individuals with CNS disorders, enhancing compliance and therapeutic outcomes.
In February 2020, Rimegepant, an ODT for acute migraine treatment, demonstrated efficacy in reducing symptoms within two hours, offering a rapid-onset option for migraine sufferers. Additionally, the development of ODT formulations for antipsychotic and antiepileptic drugs has further driven adoption in CNS treatment.
North America Dominates the Market with Strong Growth Prospects
North America leads the market with 39.2% of share in 2025, driven by the presence of well-established pharmaceutical companies, advanced healthcare infrastructure, and high healthcare expenditures. The region is projected to retain its dominance over the forecast period, primarily fueled by the rising prevalence of chronic diseases.
According to data published by the Centers for Disease Control and Prevention (CDC) on October 10, 2023, six out of ten U.S. adults suffer from chronic illnesses such as heart disease, cancer, or diabetes. Notably, heart disease and cancer alone account for approximately 38% of all deaths in the country.
Europe Emerges as the Fastest-Growing Region
Europe is the fastest-growing region in the market with 29.1% share in 2025, due to favorable reimbursement policies, a well-developed healthcare system, and a high prevalence of chronic diseases. The presence of key pharmaceutical manufacturers and continuous investments in research and development activities contribute to growth of the orodispersible tablets market in Europe.
For Instance, In December 2024, the EMA's Committee for Medicinal Products for Human Use (CHMP) issued a positive opinion recommending the approval of Leqembi for treating early-stage Alzheimer's disease. This decision came after a previous rejection due to safety concerns, marking a significant advancement in Alzheimer's treatment within the European Union.
South Asia and Oceania Lead Orally Disintegrating Tablet Market Growth, with India Emerging as a Key Country
South Asia and Oceania are witnessing rapid growth in the market, driven by multiple factors. The rising prevalence of chronic diseases has increased the need for easily accessible medications, with ODTs offering a convenient solution, particularly for individuals with swallowing difficulties. The expanding geriatric population further fuels demand for these formulations.
Advancements in pharmaceutical technology, improved healthcare infrastructure, and frequent new product launches contribute to market growth. For example, In March 2023, Zydus Lifesciences received final approval from the United States Food and Drug Administration (USFDA) to manufacture and market Olanzapine Orally Disintegrating Tablets USP, 5 mg, 10 mg, 15 mg, and 20 mg (USRLD: Zyprexa Zydis Orally Disintegrating Tablets).
The market is moderately competitive due to the presence of small and large market players who offer a range of orally disintegrating tablets. The market players are actively involved in enhancing their offering to increase their market share.
The global market is estimated to increase from US$ 25,082.1 Mn in 2025 to US$ 48,186.8 Mn in 2032.
The global market is propelled by rising demand for easy-to-administer medications, increasing geriatric and pediatric populations, advancements in drug formulations, and growing prevalence of neurological and gastrointestinal disorders.
The market is projected to record a CAGR of 8.5% during the forecast period from 2025 to 2032.
Major players include Teva Pharmaceutical Industries Ltd., Novartis AG, AstraZeneca Mylan N.V., Pfizer Inc., Johnson & Johnson Services, Inc. Others.
Opportunities include expanding pediatric formulations, innovative drug delivery technologies, rising demand for CNS drugs, and growth in emerging healthcare markets.
|
Report Attributes |
Details |
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis Units |
Value: US$ Mn, Volume: As applicable |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
|
Customization and Pricing |
Available upon request |
By Drug Class
By Disease Indication
By Distribution Channel
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author