ID: PMRREP35412| 182 Pages | 10 Jun 2025 | Format: PDF, Excel, PPT* | Healthcare

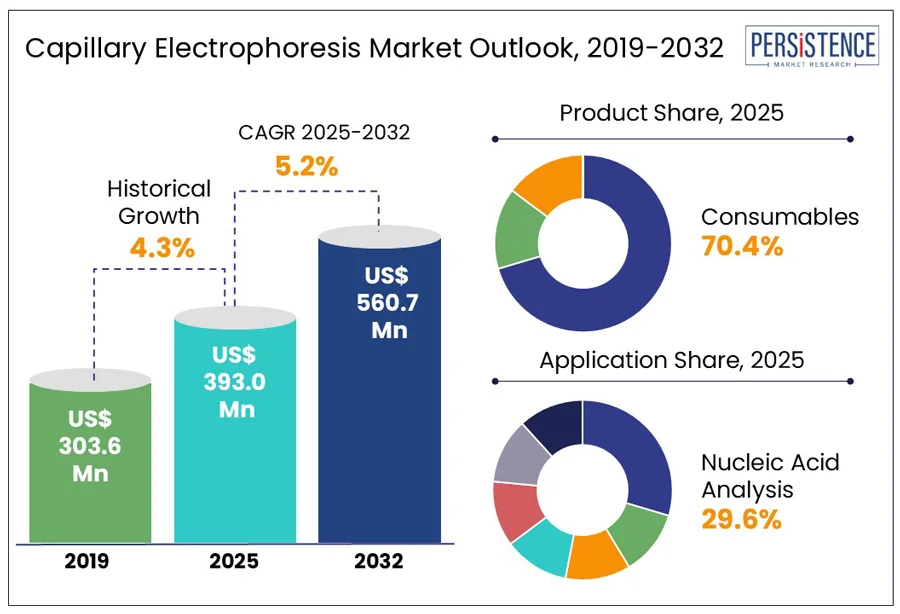
The global capillary electrophoresis market size is predicted to reach US$ 560.7 Mn in 2032 from US$ 393.0 Mn in 2025. It will likely witness a CAGR of around 5.2% in the forecast period between 2025 and 2032. Capillary Electrophoresis (CE) has transformed into a mainstay across genomics, proteomics, pharmaceutical quality control, and forensic casework. Its ability to handle minute sample volumes while rapidly delivering high-resolution separation has made it an ideal tool in laboratories striving for efficiency without compromising accuracy. The technique’s recent resurgence is driven by innovations, including capillary electrophoresis-mass spectrometry (CE-MS) integration and microfluidic CE chips.
Key Industry Highlights:
|
Global Market Attribute |
Key Insights |
|
Capillary Electrophoresis Market Size (2025E) |
US$ 393.0 Mn |
|
Market Value Forecast (2032F) |
US$ 560.7 Mn |
|
Projected Growth (CAGR 2025 to 2032) |
5.2% |
|
Historical Market Growth (CAGR 2019 to 2024) |
4.3% |
The capillary electrophoresis market growth is envisioned to grow steadily due to increasing use in paternity testing and disease diagnosis, says Persistence Market Research. In paternity testing, CE enables precise allele discrimination using fluorescently labeled Short Tandem Repeat (STR) markers. As of 2024, more than 90% of accredited private and public DNA testing laboratories worldwide use CE-based STR profiling as their key methodology owing to its legal admissibility and robustness.
In clinical diagnostics, CE has found increased utility in the detection of monoclonal gammopathies, hemoglobinopathies, and genetic mutations. The U.K.’s National Health Service (NHS), for instance, extensively uses CE-based assays to screen for sickle cell anemia and beta-thalassemia. Beckman Coulter’s P/ACE MDQ system is currently employed in multiple NHS pathology labs for routine hemoglobin variant separation. These provide rapid diagnosis within 30 minutes, which is a significant advantage in neonatal screening programs.
Capillary clogging and maintenance complexity remain significant operational limitations in the field of capillary electrophoresis. One of the core issues is the narrow internal diameter of the capillaries, which makes these prone to obstruction by aggregated proteins, residual sample debris, or precipitated buffers. This is specifically problematic in applications involving plasma or cell lysates, where sample preparation is not always optimized. As per a 2023 online survey, more than 40% of CE users reported frequent capillary blockages as a key cause of downtime in bioanalytical workflows.
Instrument downtime because of clogging not only delays analysis but can also lead to costly capillary replacement or recalibration. Instruments, including Agilent’s 7100 CE system, require reconditioning protocols involving high-pressure rinsing and pH cycling, which tend to increase maintenance time. Laboratories using outdated models or non-automated systems also lack real-time clog detection, leading to erroneous results or sample loss.
The field of forensic science is predicted to create new opportunities for CE, mainly in the analysis of STRs used for DNA profiling. CE has become the standard method for STR-based human identification as it provides high speed, resolution, and the ability to multiplex fluorescent-labeled DNA fragments. A latest development includes the adoption of the Applied Biosystems SeqStudio and 3500 series genetic analyzers by various state and federal forensic laboratories. It is because they comply with the FBI Combined DNA Index System (CODIS) standards.
Beyond human identification, CE is being applied in trace evidence analysis involving synthetic fibers, inks, and dyes. For instance, forensic labs in Germany and the Netherlands are using CE along with laser-induced fluorescence to separate and identify water-soluble ink dyes in questioned document examination. This enables discrimination between visually identical inks. This method is gaining momentum owing to its ability to work with minute sample sizes, a benefit in forensic scenarios where evidence quantity is limited.
In terms of product, the market is trifurcated into instruments, consumables, and software. Out of these, consumables are anticipated to hold approximately 70.4% of the capillary electrophoresis market share in 2025 as these represent a recurring operational necessity, unlike instruments which are one-time purchases. Each CE run requires fresh polymer matrices, capillaries, buffers, and reagents, making consumables ideal for routine analysis. As per Thermo Fisher Scientific’s 2023 product catalog, capillary arrays for the 3500 Genetic Analyzer usually require replacement every 1,000 injections, depending on use, highlighting their rising demand.
Instruments, on the other hand, are envisioned to witness a considerable CAGR from 2025 to 2032 because of their ability to serve as the central platform that enables all CE-based applications. The precision and automation capabilities embedded in modern CE instruments are important for ensuring reproducibility, specifically in regulated environments. SCIEX’s PA 800 Plus system, for instance, integrates UV detection, temperature control, and laser-induced fluorescence in a single unit. This makes it indispensable for biopharma companies to conduct glycan profiling and charge variant analysis of monoclonal antibodies.
Based on application, the market is divided into nucleic acid, protein, genomic DNA, plasmid DNA, fragment, and RNA/mRNA analysis. Among these, nucleic acid analysis is poised to hold a share of nearly 29.6% in 2025, as CE is significant for quality control of nucleic acid samples in biopharma and genomics. In Next-Generation Sequencing (NGS) workflows, CE is commonly used for sizing DNA libraries to ensure optimal fragment distribution before sequencing. Instruments, including the Bio-Rad QX200 Droplet Digital PCR platform and the Agilent Fragment Analyzer are widely used in genomics core facilities.
Protein analysis is estimated to showcase a decent growth rate through 2032 due to the ability of CE to separate proteins based on charge-to-mass ratio with high resolution. An important use case is the charge heterogeneity profiling of monoclonal antibodies (mAbs), a regulatory requirement for biologics approval. SCIEX’s PA 800 Plus system, which is used by Amgen and Genentech, is specifically designed for this purpose. SCIEX reported that more than 60% of its CE system installations in 2023 were protein-based applications.
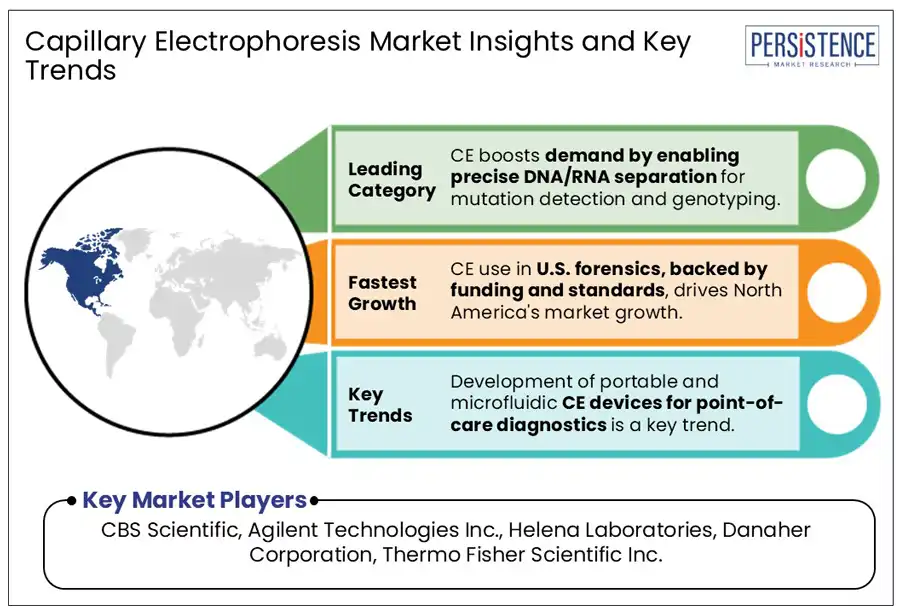
North America is projected to account for a share of around 35.7% in 2025 due to its well-established pharmaceutical manufacturing base, genomics research ecosystem, and forensic infrastructure. The U.S. capillary electrophoresis market will likely outpace Canada by 2032 amid increasing activities revolving around forensic DNA analysis and biologics characterization. The U.S. Food and Drug Administration’s high regulatory focus on gene therapies and biosimilars has propelled CE instrument upgrades and validation projects across GMP-certified labs.
Academic and government-backed genomic research is further contributing to the rising demand for CE. The Broad Institute and National Institutes of Health (NIH)-funded genome centers frequently utilize CE for library preparation QC and RNA integrity assessments. The forensic science community in the U.S. is further creating new opportunities. The FBI’s CODIS database, which continues to expand with CE-based STR profiles, uses innovative CE platforms for DNA identification.
Europe’s market is projected to be driven by increasing investment in precision medicine, expansion of the biosimilars sector, and strong regulatory frameworks for pharmaceuticals. The European Medicines Agency (EMA) mandates rigorous physicochemical characterization of biologics, including size and charge variant analysis, where CE is the gold standard. Hence, leading biopharmaceutical manufacturers in the U.K., Switzerland, and Germany have adopted CE for routine quality control.
In the Netherlands, the Netherlands Proteomics Center is employing CE-MS workflows for biomarker discovery in neurodegenerative disorders. It has been utilizing Beckman Coulter’s CE platforms integrated with Bruker MS systems. This initiative recently helped detect novel protein signatures in Alzheimer’s patients, showcasing the clinical relevance of CE beyond conventional bioanalysis. In addition, Horizon Europe projects have included CE-based analysis for environmental monitoring, thereby augmenting demand.
In Asia Pacific, South Korea, Japan, and China are showcasing steady growth due to intensified genomic research, investments in forensic infrastructure, and rapid biopharmaceutical expansion. Key China-based biopharma companies, including BeiGene and WuXi Biologics, have deployed CE systems for charge variant and glycan profiling to meet both domestic and international regulatory demands. In 2023, WuXi stated in its sustainability report that it had extended its CE capacity by over 30% to support monoclonal antibody production.
Japan is poised to be at the forefront of integrating CE with microfluidic and mass spectrometric technologies, mainly in clinical and academic proteomics. The RIKEN Institute has pioneered CE-MS platforms to analyze cerebrospinal fluid biomarkers in Parkinson’s disease. It is a part of the AMED Brain/MINDS project focuses on neurodegenerative disease diagnostics. These CE-based workflows are being adopted in parallel by precision medicine consortia in South Korea, primarily under the Korea Bio-Bank Project.
The global capillary electrophoresis market houses a few leading companies and niche players focused on specialized applications. Key players are providing innovative CE systems with integrated software and automation. They are benefiting from long-term collaborations with academic institutions and regulatory agencies as well as robust distribution networks. Niche companies are striving to gain a competitive advantage through miniaturization, applications in point-of-care diagnostics, and integration with mass spectrometry.
The market is projected to reach US$ 393.0 Mn in 2025.
Increasing use in forensic casework and rising demand for paternity tests in specific countries are the key market drivers.
The market is poised to witness a CAGR of 5.2% from 2025 to 2032.
Investments in precision medicine and surging genomics research activities are the key market opportunities.
CBS Scientific, Agilent Technologies Inc., and Helena Laboratories are a few key market players.
|
Report Attribute |
Details |
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis Units |
Value: US$ Bn/Mn, Volume: As Applicable |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
|
Customization and Pricing |
Available upon request |
By Product
By Mode
By Application
By End-use
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author