ID: PMRREP30383| 191 Pages | 8 Sep 2025 | Format: PDF, Excel, PPT* | Healthcare

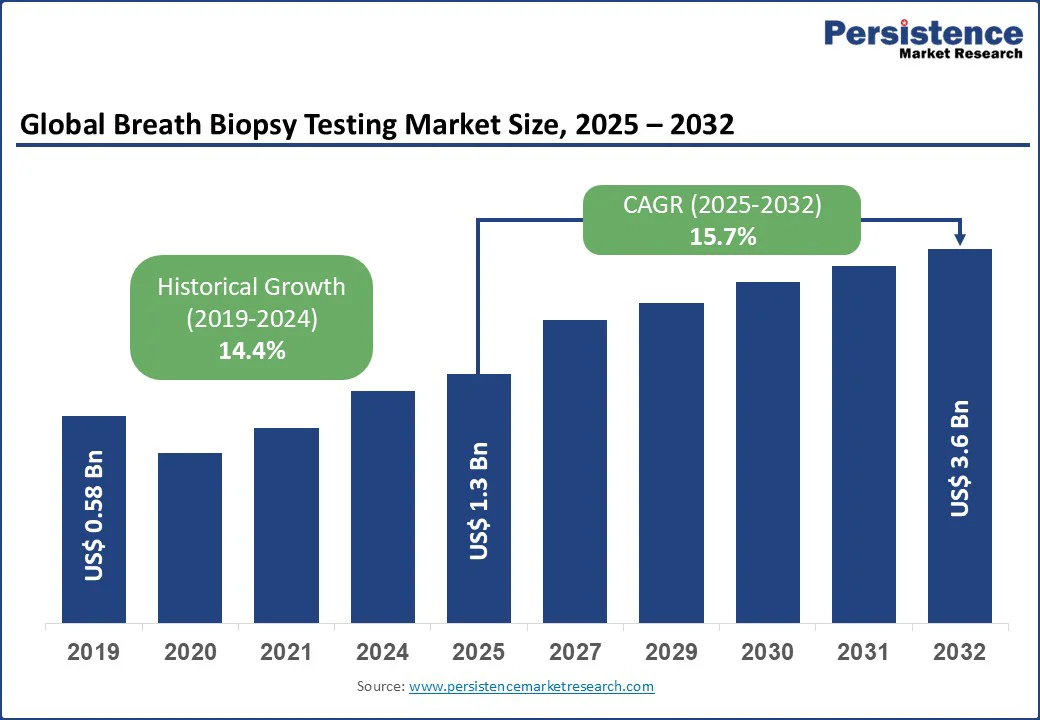
The global breath biopsy testing market size is likely to value at US$1.3 Bn in 2025 to US$3.6 Bn by 2032, registering a CAGR of 15.7% during the forecast period from 2025 to 2032.
The breath biopsy testing market has experienced robust growth, driven by the rising prevalence of chronic and infectious diseases, advancements in biomarker detection technologies, and increasing demand for non-invasive diagnostic solutions. The expansion is further supported by global efforts to combat cancer and respiratory illnesses, and improve healthcare access in developing regions.
Key Industry Highlights:
|
Global Market Attribute |
Key Insights |
|
Breath Biopsy Testing Market Size (2025E) |
US$1.3 Bn |
|
Market Value Forecast (2032F) |
US$3.6 Bn |
|
Projected Growth (CAGR 2025 to 2032) |
15.7% |
|
Historical Market Growth (CAGR 2019 to 2024) |
14.4% |
The global surge in chronic diseases is a primary driver of the breath biopsy testing market. According to the World Health Organization, cancer accounted for nearly 20 million new cases and 9.7 million deaths worldwide in 2022, with lung cancer being a leading cause, responsible for about 2.5 million new cases annually. The rising incidence, particularly in aging populations and urban areas with high pollution levels, underscores the urgent need for non-invasive diagnostics such as breath biopsy, which can detect volatile organic compounds (VOCs) linked to metabolic changes in diseases.
In 2024, the American Cancer Society reported over 2 million new cancer diagnoses in the U.S., highlighting the demand for early detection tools that breath biopsy provides, especially for lung cancer where survival rates improve significantly with early intervention.
Technological advancements in breath analysis systems are significantly boosting market growth. Modern platforms, such as Owlstone Medical’s Breath Biopsy system, offer high sensitivity and specificity, reducing false negatives and enabling faster diagnosis through real-time VOC profiling. Clinical studies have shown that breath-based tests can reduce diagnostic turnaround time by nearly half compared to traditional biopsy methods, with accuracy rates above eighty percent for lung cancer detection. Innovations such as portable spectrometers and AI-integrated data analysis further enhance adoption, particularly in resource-limited settings where invasive procedures are challenging.
Government initiatives and increased funding for non-invasive diagnostics are also key growth drivers. In the U.S., programs from the National Cancer Institute supporting biomarker research have expanded access to breath testing, increasing demand for advanced kits. In Europe, the Horizon Europe program backs research into breath biomarkers, while in Asia, initiatives such as China’s Healthy China plan encourage investment in innovative diagnostics. Favorable reimbursement policies in North America for non-invasive tests, coupled with heightened surveillance for respiratory diseases post-COVID-19, encourage healthcare facilities to invest in breath biopsy equipment.
The high cost of breath biopsy testing kits remains a significant barrier to widespread adoption, particularly in low- and middle-income countries. Advanced diagnostic platforms, equipped with features such as high-resolution mass spectrometry and real-time data integration, require substantial upfront investment. Additionally, ongoing costs for calibration, sensors, and quality control add to the total cost of ownership. In regions such as Sub-Saharan Africa and rural parts of South Asia, where healthcare budgets are constrained, these financial burdens limit access to breath biopsy, even amidst rising chronic disease burdens.
The World Health Organization has highlighted that “the cost of advanced diagnostic tests may be a disincentive,” highlighting high diagnostic costs as a barrier to widespread adoption of innovative tools in many countries.
The requirement for skilled personnel to operate and interpret breath biopsy systems also hinders market growth. Analyzing VOC profiles or mass spectrometry data demands specialized training for laboratory technicians. A shortage of certified experts in breathomics in developing regions exacerbates this challenge. This skills gap, combined with high training costs, restricts the adoption of advanced systems in emerging markets, slowing market expansion.
The development of portable and compact breath biopsy testing kits presents significant growth opportunities, enabling deployment in remote clinics, mobile health units, and emergency screening scenarios. These portable systems overcome the limitations of traditional invasive diagnostics, making them ideal for decentralized healthcare settings. For example, Owlstone Medical’s ReCIVA breath sampler offers rapid VOC capture within minutes, supporting its use in rural and field settings.
As healthcare systems prioritize accessible diagnostics, demand for such solutions is rising, particularly in regions with limited laboratory infrastructure.
The growing popularity of point-of-care (POC) testing, such as electronic nose (e-nose) devices for breath analysis, provides another avenue for market expansion. These tests require minimal equipment and deliver results quickly, making them suitable for resource-constrained environments. Clinical studies have shown that POC breath tests significantly reduce diagnosis-to-treatment time compared to traditional methods, driving demand for innovative kits in disease-prone areas such as lung cancer hotspots.
The integration of digital health platforms for remote monitoring and data sharing further enhances market potential. Companies such as IONICON are incorporating IoT-enabled diagnostics into their systems, allowing real-time data transmission and proactive analysis. This trend improves accessibility and operational efficiency, supporting market growth in both developed and emerging regions.
The global breath biopsy testing market is segmented into volatile organic compounds analysis, mass spectrometry, colony counting, and gas chromatography. Volatile organic compounds analysis dominates, holding approximately 35.50% of the breath biopsy testing market share in 2025, due to its critical role in detecting metabolic biomarkers during the early stages of diseases such as cancer. Advanced VOC analysis tools, such as Owlstone Medical’s platforms, are widely adopted for their ease of use and rapid results, making them essential in screening settings.
Mass spectrometry is the fastest-growing segment, driven by increasing demand for high-accuracy diagnostics in research and clinical settings. Innovations in high-resolution mass spectrometry technologies, such as MKS Instruments’ systems, offer superior sensitivity and specificity, boosting adoption in high-volume diagnostic centers.
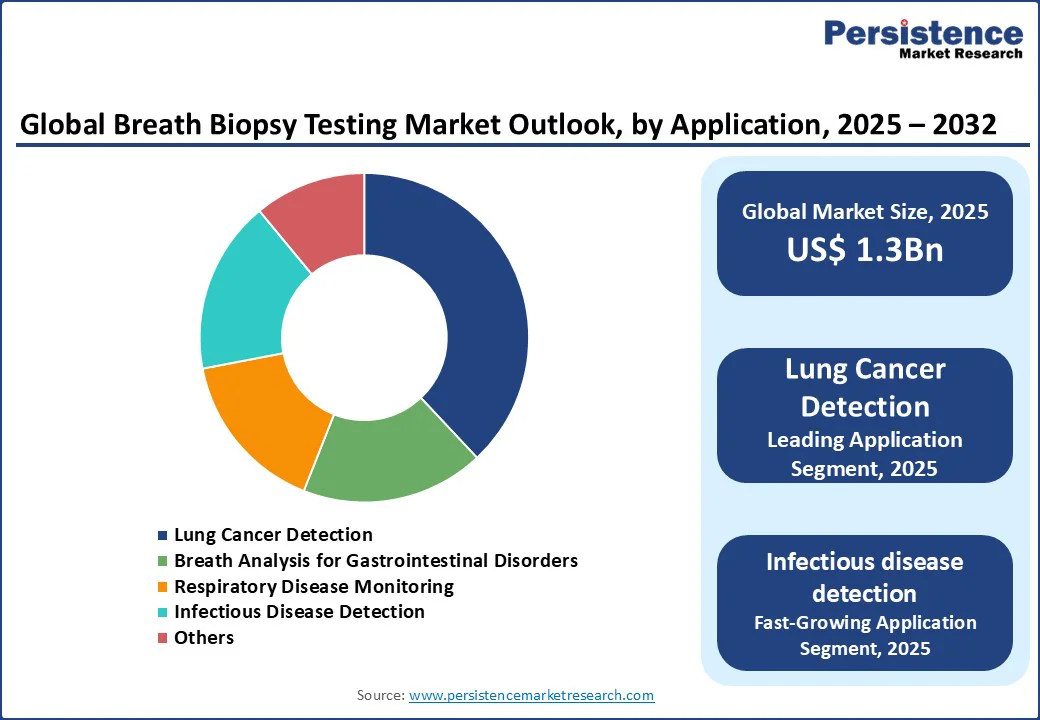
The global breath biopsy testing market is divided into lung cancer detection, breath analysis for gastrointestinal disorders, respiratory disease monitoring, and infectious disease detection. Lung cancer detection leads with a 38% share in 2025, driven by its widespread use in oncology and clinical settings. These applications, which identify cancer-specific VOCs, are critical for early intervention, with over 500,000 tests performed annually worldwide for lung cancer screening.
Infectious disease detection is the fastest-growing segment, fueled by advancements in breath-based pathogen identification and the rising prevalence of respiratory infections. Its ability to confirm infections with high precision drives its adoption in advanced diagnostic facilities, particularly for epidemiological studies post-global pandemics.
The global breath biopsy testing market is segmented into exhaled breath, breath condensate, and sweat and dermal samples. Exhaled breath dominates with a 55% share in 2025, driven by its high sampling volumes and non-invasive nature. This sample type relies on simple collection methods to capture VOCs, making it essential for routine screening in hospitals and labs.
Breath condensate is the fastest-growing segment, propelled by the increasing focus on detailed biomarker analysis for complex diseases. These samples, which allow for concentrated metabolite detection, cater to the growing demand for high-throughput testing and research applications.
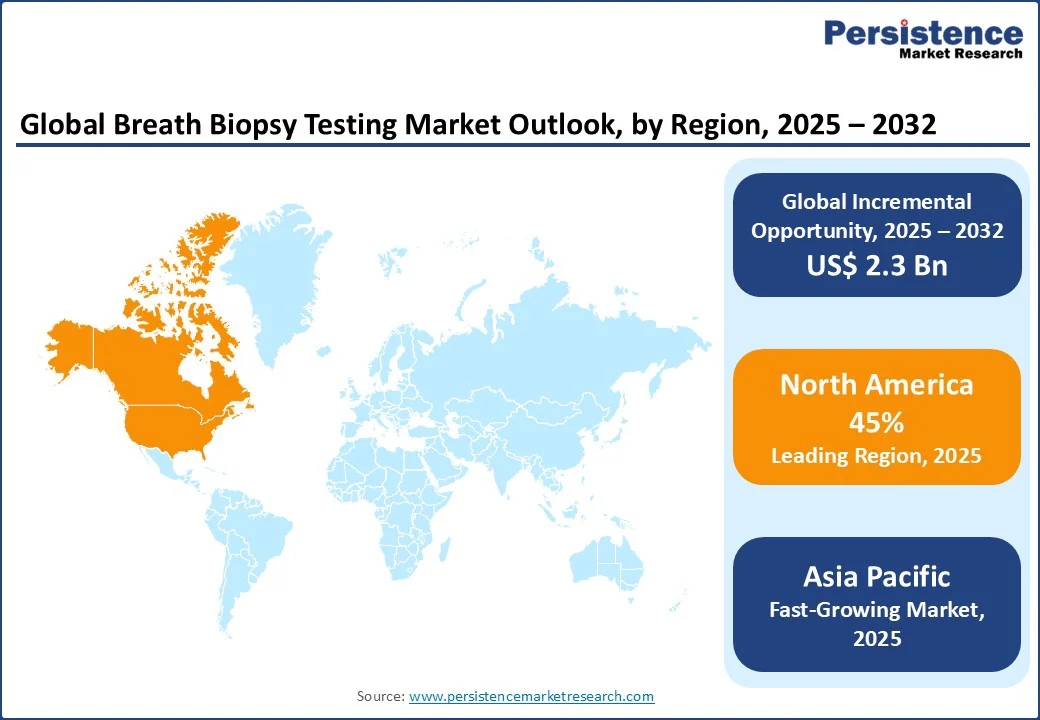
In North America, the U.S. is dominant and is expected to account for a 45% share in 2025. This dominance is driven by high surveillance for chronic diseases, advanced diagnostic infrastructure, and increasing cases of lung cancer and respiratory illnesses. The CDC reports a substantial number of new lung cancer cases annually, underscoring the urgent need for robust non-invasive testing solutions. To address this demand, leading brands such as Owlstone Medical and Teledyne Tekmar are developing innovative platforms designed to support diagnostic teams with accuracy, speed, and patient-friendly methods.
Consumer preferences are shifting toward compact, automated testing systems with AI-integrated analysis, such as Aeroqual’s breath analyzers, which enhance diagnostic accuracy and speed. Stringent FDA regulations prioritize patient safety, encouraging the adoption of reliable, high-sensitivity components. Favorable reimbursement policies for non-invasive tests further incentivize hospitals and labs to invest in advanced equipment, supporting market growth.
Europe’s breath biopsy testing market is led by Germany, the U.K., and France, driven by regulatory support and high diagnostic volumes. Germany holds the largest share, supported by strong sales from companies such as IONICON and MKS Instruments. The EU’s Medical Device Regulation (MDR) fosters innovation and compliance, promoting the adoption of advanced VOC and mass spectrometry systems in major healthcare facilities.
In the U.K., the rising demand for point-of-care diagnostics, with products such as Owlstone Medical’s breath biopsy kits gaining popularity for their precision and portability. France is witnessing increased demand for cancer-related diagnostics, with Ambetronics Engineers Pvt. Ltd. offering specialized solutions. Regulatory support for sustainable manufacturing practices across Europe further enhances market prospects.
Asia Pacific represents the fastest-growing market for breath biopsy testing, driven by expanding healthcare infrastructure, increasing disease prevalence, and rising investments in diagnostic technologies. India remains a key growth engine, where rising cancer rates and government programs such as the Ayushman Bharat scheme are boosting demand for affordable, semi-automated testing solutions. Domestic manufacturers such as Uniphos Envirotronic Pvt. Ltd. and Vasthi Engineers Pvt Ltd. cater to local needs with cost-effective kits designed for both urban and rural healthcare settings.
In China, rapid market expansion is supported by large-scale laboratory upgrades, growing adoption of automated breath testing systems, and the presence of leading players such as Yokogawa India. Japan’s market is characterized by demand for high-precision diagnostic tools used in research and epidemiological surveillance, with companies such as Aeroqual gaining market share. Across the region, increased healthcare spending, digital procurement platforms, and the emphasis on early disease detection are collectively accelerating adoption, making the Asia Pacific a critical hub for future market growth.
The global breath biopsy testing market is highly competitive, with global and regional players vying for market share through innovation, competitive pricing, and reliability. The rise of portable and automated testing systems intensifies competition, as companies strive to meet stringent regulatory standards and diagnostic demands. Strategic partnerships, mergers, and regulatory approvals are critical differentiators in this dynamic market.
The breath biopsy testing market is projected to reach US$1.3 Bn in 2025.
Rising chronic diseases, technological advancements in non-invasive diagnostics, and government healthcare initiatives are the key market drivers.
The breath biopsy testing market is poised to witness a CAGR of 15.7% from 2025 to 2032.
Innovations in portable testing systems and point-of-care diagnostics present significant growth opportunities.
Owlstone Medical, IONICON, and MKS Instruments are among the leading market players.
|
Report Attribute |
Details |
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis |
Value: US$ Bn Volume: As Applicable |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
By Test Type
By Application
By Sample
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author