ID: PMRREP16075| 192 Pages | 7 Jun 2017 | Format: PDF, Excel, PPT* | Healthcare

Persistence Market Research forecasts the global endoscope repair market to grow from US$ 976.8 Mn in 2016 to about US$ 1,650 Mn by 2025 end. This represents a CAGR of 6.0% over the forecast period 2017–2025. The global market for endoscope repair is estimated to represent absolute dollar opportunity of US$ 66.6 Mn in 2018 over 2017 and incremental opportunity of US$ 613 Mn between 2017 and 2025.
Increasing demand for minimally invasive procedures – also known as endoscopic procedures - will have a high impact across the forecast period. This can be attributed to the increasing incidence of diseases and an ageing population. Minimally invasive procedures have several advantages for doctors as well as patients. Minimally invasive procedures are safer as compared to open surgeries as they involve small incisions in the patient’s body, unlike open procedures.
The advantages include reduced blood loss, lower chances of infection, faster recovery time and fewer surgery marks on the body. Minimally invasive procedures reduce the risk of atrial fibrillation and are less time consuming. These advantages have garnered a lot of consumer attention and hence drive the endoscope repair market. The trend of a rising demand for endoscopic procedures is expected to lead to growth of the endoscope repair market over the forecast period.
The rising prevalence of colorectal and gastrointestinal diseases has resulted in an increase in endoscopy procedures for detection and treatment purposes. According to the World Health Organization (WHO), the incidences of benign, malignant gastrointestinal (GI) diseases and colorectal complications are rising worldwide in elder patients. As per WHO, in 2008, elder patients constituted the greatest proportion of colorectal cancer diagnosis with an incident rate of 247.6/100,000, esophageal cancer diagnosis with an incident rate of 23.3/100,000 and gastric cancer diagnosis with an incidence rate of 40.8/100,000.
The elderly have also increased incidences of other diseases such as pancreatic and biliary diseases with gallstones. Thus, for the detection of cancer and tumors, doctors and patients prefer endoscopes due to the safety and efficacy of endoscopic procedures. This propels the demand for endoscopes and drives growth of the endoscope repair market.
Inadequate healthcare infrastructure in emerging markets may restrain growth of the endoscope market. The lack of proper development and implementation of clinical practice guidelines and dearth of skilled labor in developing countries such as China, India and Middle East and African countries hampers the demand and application of specialized endoscopy devices and hence hinders growth of the market. Lack of a proper regulatory framework in emerging markets is also hindering revenue growth of the endoscope repair market.
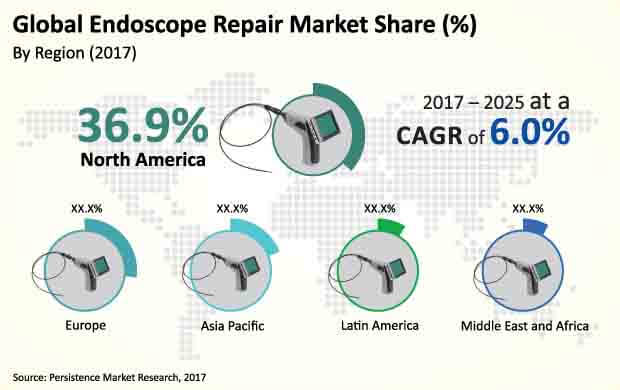
North America dominated the global endoscope repair market in terms of revenue in 2017, and the trend is projected to continue throughout the forecast period. North America is the most attractive market, with an attractiveness index of 1.8 over the forecast period.
Revenue from the North America endoscope repair market is anticipated to increase at a CAGR of 5.9% over 2017–2025, to reach more than US$ 605 Mn by 2025. Europe is expected to be the second most lucrative market in the global endoscope repair market with an attractiveness index of 1.4 during the forecast period.
In endoscopic procedures, the diagnosis and treatment of diseases is done through natural openings of the body and sometimes through small incisions taken on the body. Attributing to the presence of small or no incisions, the chances of developing an infection are reduced, as compared to conventional procedures where large incisions are required and the chances of infection are more. Endoscopic procedures also reduce healthcare expenditure significantly.
These procedures have a short procedure time and a low risk of complications and hence, patients have a shorter hospital stay with lower pre- and post-operative costs. These benefits have resulted in the growing acceptance of endoscopic procedures, which is anticipated to boost demand and drive growth of the global endoscope repair market.
| Attribute | Details |
|---|---|
|
By Product Type |
|
|
By Modality Type |
|
|
By Service Provider |
|
|
By Facility Type |
|
|
By Region |
|
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author