ID: PMRREP15877| 180 Pages | 11 May 2017 | Format: PDF, Excel, PPT* | Chemicals and Materials

Global sales of chromatographic silica resin are estimated to be valued at more than US$80 million by the end of 2017. They are expected to increase to more than US$110 Mn by 2025, representing an incremental opportunity of more than US$30 Mn between 2017 and 2025. Global sales revenue of chromatographic silica resin is projected to exhibit a CAGR of 4.1% during the forecast period.
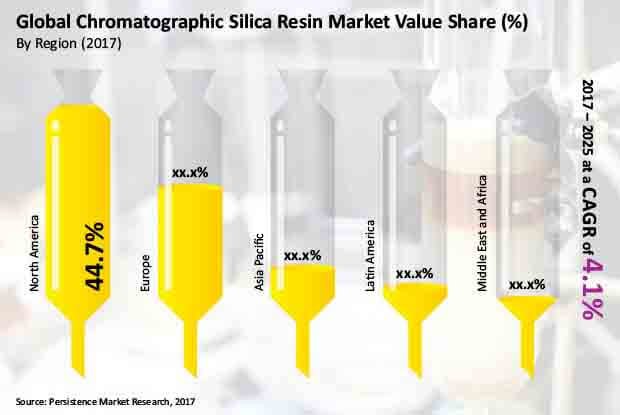
In terms of regional markets, North America is estimated to be the major market accounting for over 40% of the total market share. In contrast, the Asia Pacific is projected to be a high-growth market over the forecast period.
The stable growth of the pharmaceutical and biotechnology industries is expected to boost the global chromatographic silica resin consumption over the forecast period. In the pharmaceutical industry, silica gel column chromatography is used to separate and collect the different components of a drug.
This has application in the purification of antibiotics such as rosarimicin, coloradocin and benzanthrins, among others. Silica resin chromatography is also used in the study of medicines such as hypnotics, sedatives, analgesics, local anesthetics and steroids.
Chromatography also finds use in the field of biotechnology where it is used due to its ability to detect molecular components such as nucleic acids, fats, carbohydrates, protein and vitamins.
Protein is the most desired component in a number of medicines and supplements and holds a high degree of importance in biopharmaceuticals. Size-exclusion chromatography is widely used for the purification and analysis of synthetic and biological polymers.
Chromatographic silica resin is used in thin-layer chromatography for biomedical analysis. There is a growing demand for medicines, especially in developing economies such as China and India, and this is expected to drive the demand for biopharmaceuticals, which in turn will drive the growth of the chromatographic silica resin market during the assessment period.
There is a growing problem of adulteration of hazardous ingredients and cheap substitutes in food items. However, in order to ensure the good quality and consistency of food products, clinical trials are conducted during the development of new food products as well as during existing batch production of food products. Chromatography is an important method in checking adulteration in food products.
In the food industry, chromatography is widely used in a number of applications such as vitamin separation, sugar content analysis, triglyceride determination, determination of nutritional quality of food, spoilage detection, analysis of amino acids, colorants and residues, detection of aflatoxins in food products and many more. Hence, the use of chromatographic silica resin in the food industry is expected to witness a steady growth owing to a pulsating food industry in countries such as India.
Activated alumina is famous for its superiority in separation and purification techniques. Due to the atmospheric properties of alumina, it can be used with neutral, acidic and basic compounds as well.
On the other hand, silica gel can only be used in limited end use industries whereas alumina, being a superior ingredient, is used for environmental cleanup and in many other applications such as biohazard, bioterrorism, gas and liquid dehydration applications. Due to its versatile applications at a relatively lower cost, alumina resins are a preferred choice over silica resins. These factors may restrict the growth of the chromatographic silica resin market during the assessment period.
North America region is expected to emerge as a relatively attractive market in terms of incremental dollar opportunity by the end of forecast period. Asia Pacific is expected to grow at a significant CAGR as compared to other regions.
Increasing demand for chromatography from end-use industries as a result of economic growth, especially in emerging markets such as China and India drives the APAC chromatographic silica resin market. In terms of market value, the Europe chromatographic silica resin market is estimated to account for 31.5% share by 2017 end, whereas Latin America will account for 5.7% share.
Due to the stable economic growth in emerging economies such as China and India, a good number of opportunities will be created for the chromatographic silica resin market during the assessment period. There is a huge demand for pharmaceutical products in these countries.
Moreover, the demand for chromatographic resins like silica gel is also increasing in agriculture, food and beverage and biotechnology industries as well as in academic institutes and R&D labs.
In addition, tremendous growth in China and India has resulted in increased government expenditure on healthcare and biomedical industries. In addition, food security concerns in India have prompted the government to invest hugely in agriculture and soil testing.
Similarly, strategic expansion of chromatography players in China can be a boon for the chromatographic silica resin market. Also, the presence of bio-clusters in these countries may also propel the chromatographic silica resin market in the near future.”
--- Analyst – Chemicals and Nanomaterials, Persistence Market Research
|
By Mesh Size |
|
|
By Purity |
|
|
By Application |
|
|
By End Use |
|
|
By Region |
|
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author